In vitro assays for residual pluripotent stem cells in derived cell therapy products: what’s the current state of play?
Pluripotent cell therapies require stringent quality control testing. In this article, Sistemic (Glasgow, UK) discuss the current challenges and options.

Introduction
Pluripotent stem cells (PSCs) — such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) — are a type of stem cell that can be transformed into any cell type in the body. PSC-derived therapies have great promise in the effective treatment of many human diseases, potentially providing curative medical solutions for a wide range of life-threatening diseases, including cancer, cardiovascular, metabolic, immune system & neurological disease [1-3]. As such, these cellular therapies have the capacity to be transformational. There is an increasing number of cell therapy and pharmaceutical companies that are developing PSC-derived cellular therapies, with some in clinical development [1, 4-7] and showing promising indications of clinical efficacy [4-7].
Nevertheless, a critical safety issue in the development of these potentially transformational therapies is the possibility of residual undifferentiated PSCs persisting in the final cell therapy product, which can give rise to teratomas or other neoplasms [8, 9]. A safety assay to quality control (QC) the levels of residual undifferentiated PSCs is required by regulatory agencies to confirm purity of the final product [10-12].
While a specific approach is not defined or promoted by regulatory agencies, QC assay data packages included in applications for regulatory approval are reviewed on a case-by-case basis [10-12]. There are ongoing public—private initiatives to establish internationally harmonized technology platforms and assays [10, 11]. However, there are currently no globally accepted and established in vivo or in vitro methods for assessment of residual PSC levels [10, 11]. Nonetheless, there is an increasing recognition that an in vitro off-the-shelf assay for QC testing would be highly beneficial to the continued successful clinical development and safe delivery of PSC-derived therapies to patients [11].
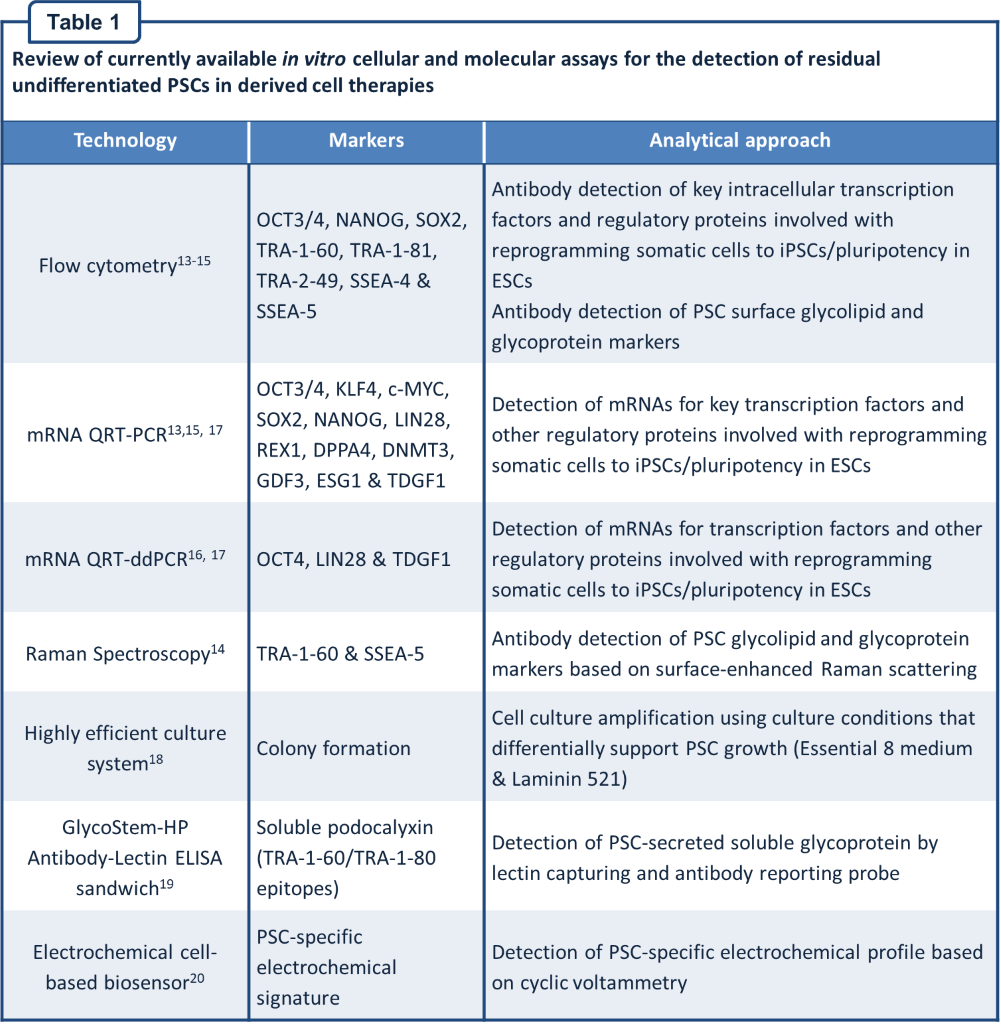
Review of currently available in vitro cellular and molecular approaches
A variety of technology platforms and assays have been reported for the in vitro detection of residual undifferentiated PSCs in derived cell therapies. Details of the technology, markers and analytical approach for these assays are given in Table 1.
What are the requirements for an in vitro safety QC assay for residual undifferentiated PSCs?
There are three key performance and regulatory requirements that need to be considered and addressed for an in vitro safety QC assay to test residual undifferentiated PSCs for a PSC-derived cell therapy.
Detection sensitivity
In this developing field, the detection sensitivity requirements are still being defined. Currently, regulatory agencies indicate that they are being determined on a case-by-case basis according to a risk-based approach [10-12]. However, current consensus appears to be that an assay should be capable of detecting residual undifferentiated PSC levels to at least 0.001% sensitivity i.e. 10 PSCs in a background of 1×106 derived cells [10, 11].
Diagnostic accuracy
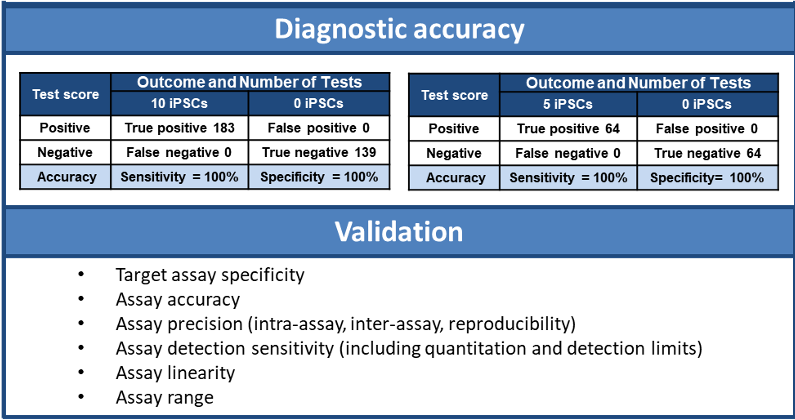
Diagnostic accuracy is an overall assessment of an assay’s ability to identify a true positive and a true negative in all cases. This can be determined from the assay analytical sensitivity (i.e. the proportion of true positives that test positive) and the analytical specificity (i.e. the proportion of true negatives that test negative) [21].
It is important to assess and understand the diagnostic accuracy for this type of safety assay. At the present time, diagnostic accuracy requirements are not defined; however, given that PSCs have a relatively high inherent risk of tumor formation [10, 11], it is likely that regulatory agencies would be looking for 100% analytical specificity (zero false negative rate).
“Sistemic’s SistemPSCCheckâ„¢ 1.0 assay was developed and optimized to specifically meet the key assay performance and regulatory requirements for detection sensitivity, diagnostic accuracy and validation.“
On the other hand, manufacturers would presumably require 100% analytical sensitivity, or zero false positive rate, therefore saving the costs of unnecessarily discarding manufactured cell therapy products. Accordingly, it is reasonable to assume that a QC safety assay for detecting residual undifferentiated PSCs would need to have 100% diagnostic accuracy with no false negatives and no false positives.
Validation
All aspects of assay performance need to be systematically evaluated and validated according to established International Conference on Harmonisation (ICH) Validation of Analytical Procedures guidelines for assay validation dubbed Q2(R1) [22]. This is required by regulatory agencies [11, 12].
Introducing SistemPSCCheckâ„¢1.0
Background and assay details
In 2018, Sistemic recognized the unmet need for a highly sensitive and validated safety QC assay to assess residual undifferentiated PSC levels in derived cell therapy products. In order to support the clinical development of these cutting-edge therapies, Sistemic began development of SistemPSCCheckâ„¢ 1.0. This was carried out in conjunction with the UK National Measurement Laboratory for Chemical and Bio-Measurement (NML, hosted at LGC in Teddington, UK) and supported by Innovate UK (Swindon, UK). The SistemPSCCheckâ„¢ 1.0 assay was developed and optimized using human iPSCs as the PSC source and human bone marrow-derived mesenchymal stem cells (BM-MSCs) as the background tissue-derived equivalent of a PSC-derived MSC.
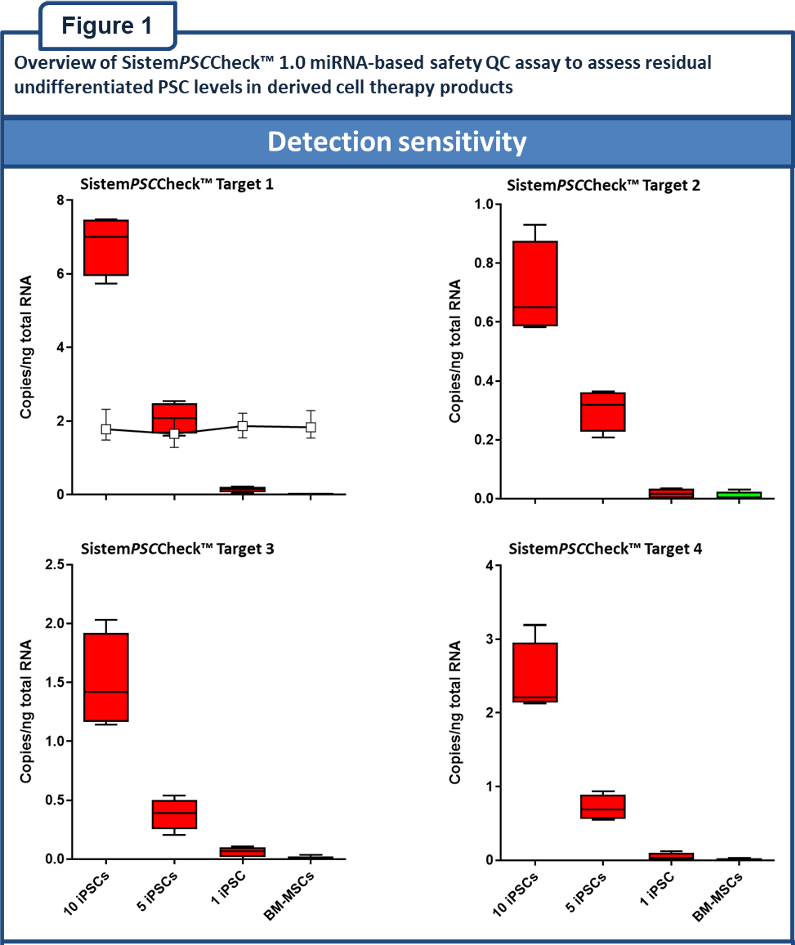
“SistemPSCCheckâ„¢ 1.0 is a first-in-class miRNA-based droplet digital (dd)PCR safety QC assay for the highly sensitive and quantitative assessment of residual undifferentiated PSC levels in derived cell therapy products.”
The assay comprises expression analysis of a panel of four miRNA targets. The copy number of the miRNA targets correlates with PSC numbers. Using this relationship, the assay quantitatively establishes the number of residual undifferentiated PSCs in derived cell preparations.
“The SistemPSCCheckâ„¢ 1.0 assay has a detection sensitivity of ≤0.0005%, a diagnostic accuracy of 100% and has been validated according to ICH Q2(R1) guidelines [Figure 1]”
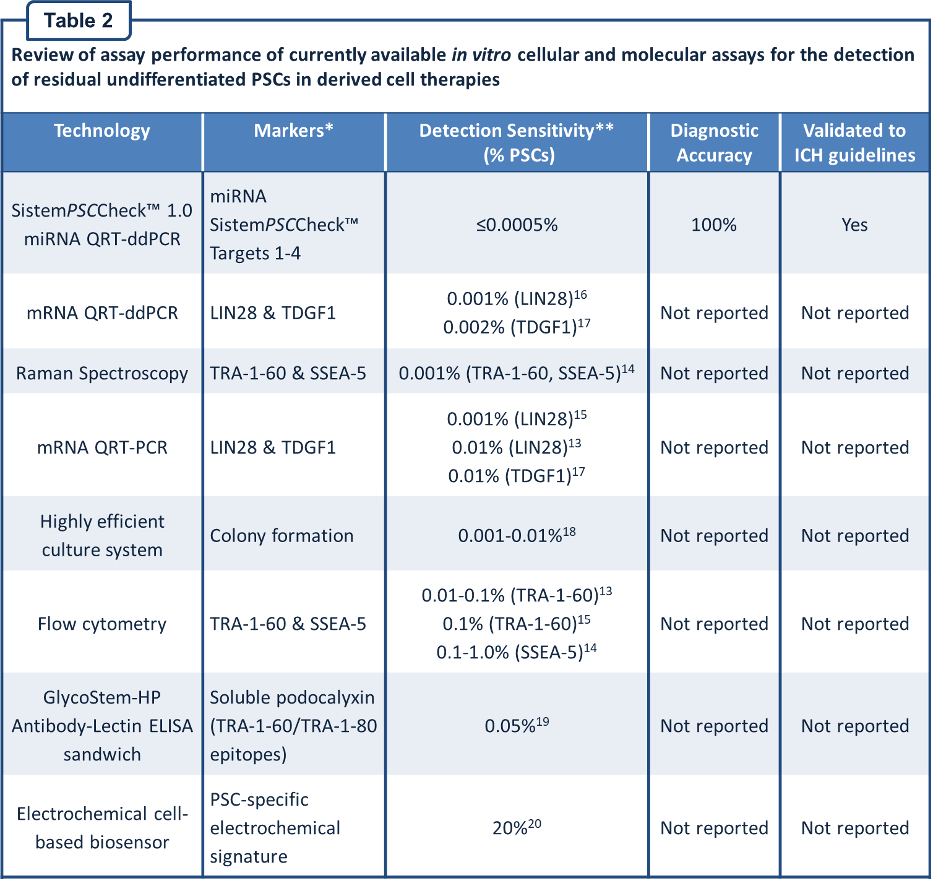
Review of assay performance of currently available in vitro cellular and molecular assays compared with SistemPSCCheckâ„¢ 1.0
The assay performance of currently available in vitro cellular and molecular assays is compared with the SistemPSCCheckâ„¢ 1.0 assay below (Table 2). From the information detailed here, it is clear that SistemPSCCheckâ„¢ 1.0 outperforms other in vitro cellular and molecular assays that are available at the present time.
Summary
- A validated safety assay to QC the levels of residual undifferentiated PSCs is required by regulatory agencies to confirm purity of the final product.
- An in vitro off-the-shelf assay, suitable for routine QC testing, would be highly beneficial to the continued and successful clinical development and safe delivery of PSC-derived therapies to patients.
- The SistemPSCCheckâ„¢ 1.0 assay was developed and optimized to specifically meet the key assay performance and regulatory requirements for detection sensitivity, diagnostic accuracy and validation.
- The SistemPSCCheck™ 1.0 assay has a detection sensitivity of ≤0.0005%, a diagnostic accuracy of 100% and has been validated according to ICH Q2(R1) guidelines.
- SistemPSCCheckâ„¢ 1.0 is the only available off-the-shelf in vitro assay for assessment of residual undifferentiated PSC levels in derived cell therapy products and clearly outperforms the currently available alternative in vitro cellular and molecular assays.
Further information
For potential partners interested in receiving a fully detailed validation data dossier, further information, or to be part of an early access program, please contact [email protected].
Acknowledgements
Sistemic Ltd. would like to acknowledge the support of the UK’s innovation agency, Innovate UK, under “The Analysis for Innovators” grant (TSB 103717), and the NML for their work involving developing the assay.
References
- Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 17(1), 11—22 (2015).
- Glicksman M. Induced pluripotent stem cells: the most versatile source for stem cell therapy. Clin. Ther. 40(7), 1060—1065 (2018).
- Bragança J, Lopes J, Mendes-Silva L, Santos J. Induced pluripotent stem cells, a giant leap for mankind therapeutic applications. World J. Stem Cells. 11(7), 421—430 (2019).
- Zarbin M, Sugino I, Townes-Anderson E. Concise review: update on retinal pigment epithelium transplantation for agerelated macular degeneration. Stem Cells Transl. Med. 8(5), 466—477 (2019).
- Lineage Cell Therapeutics. Lineage Cell Therapeutics provides update on SCiStar clinical study and OPC1 spinal cord injury program www.investor.lineagecell.com/n… Accessed 7 February 2020.
- Rasko J, Patel A, Griffin J, et al. Results of the first completed clinical trial of an iPSC-derived product: CYP-001 in steroid-resistant accute GvHD. Biol. Blood Marrow Transplant. 25(3), S255—S256 (2019).
- Lineage Cell Therapeutics. Lineage Cell Therapeutics provides update on SCiStar clinical study and OPC1 spinal cord injury program www.investor.lineagecell.com/n… Accessed 4 February 2020.
- Herberts C, Kwa M, Hermsen H. Risk factors in the development of stem cell therapy. J. Transl. Med. 9, 29 (2011).
- Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer. 11(4), 268—277 (2011).
- Abbot S, Agbanyo F, Ahlfors JE et al. Report of the international conference on manufacturing and testing of pluripotent stem cells. Biologicals. 56, 67—83 (2018).
- Sato Y, Bando H, Piazza M et al. Tumorigenicity assessment of cell therapy products: the need for global consensus and points to consider. Cytotherapy. 21(11), 1095—1111 (2019).
- UK Medicines & Healthcare Regulations Agency, Innovation Office. www.gov.uk/government/groups/m… Accessed 22 June 2018
- Kuroda T, Yasuda S, Kusakawa S et al. Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells. PLoS One. 7(5), e37342 (2012).
- Han J, Qian X, Wu Q et al. Novel surface-enhanced Raman scattering-based assays for ultra-sensitive detection of human pluripotent stem cells. Biomaterials. 105, 66—76 (2016).
- Ito E, Miyagawa S, Takeda M et al. Tumorigenicity assay essential for facilitating safety studies of hiPSC-derived cardiomyocytes for clinical application. Sci. Rep. 9(1), 1881 (2019)
- Kuroda T, Yasuda S, Matsuyama S et al. Highly sensitive droplet digital PCR method for detection of residual undifferentiated cells in cardiomyocytes derived from human pluripotent stem cells. Regen Ther. 2, 17—23 (2015).
- Artyuhov A, Dashinimaev E, Mescheryakova N, Ashikhmina A, Vorotelyak E, Vasiliev A. Detection of small numbers of iPSCs in different heterogeneous cell mixtures with highly sensitive droplet digital PCR. Mol. Biol. Rep. 46, 6675—6683 (2019).
- Tano K, Yasuda S, Kuroda T, Saito H, Umezawa A, Sato Y. A novel in vitro method for detecting undifferentiated human pluripotent stem cells as impurities in cell therapy products using a highly efficient culture system.PLoS One. 9(10), e110496 (2014).
- Tateno H, Hiemori K, Hirayasu K et al. Development of a practical sandwich assay to detect human pluripotent stem cells using cell culture media. Regen Ther. 6, 1—8 (2017).
- Yea C, Jeong H, Moon S et al. In situ label-free quantification of human pluripotent stem cells with electrochemical potential. Biomaterials. 75, 250—259 (2016).
- Å imundić A. Measures of diagnostic accuracy: basic definitions. EJIFCC. 19(4), 203—211 (2009).
- International Conference on Harmonization. Q2(R1): Validation of Analytical Procedures: Text and Methodology, 2005.
About Sistemic Ltd.
Sistemic is an award-winning innovative small/medium sized enterprise based in Glasgow, UK. Sistemic’s global business is focused on providing pioneering microRNA (miRNA)-based tools, services and products for areas of unmet need within stem cell therapy, cell therapy and regenerative medicine, and to support the development of novel therapies in these markets. Sistemic has a global customer base and, over the past 10 years, has worked with over 65 leading cell therapy companies, contract manufacturing organizations and research organizations supporting the development of their cell therapy products.