Release of Human Embryonic Stem Cell Lines Suitable for the Manufacture of Advanced Therapies
The UK Stem Cell Bank is releasing ‘EUTCD-Grade’ cell lines deemed suitable for the manufacture of advanced therapies (under EU law) which are to be brought to clinical trial
For the first time the UK Stem Cell Bank (UKSCB) is releasing human embryonic stem cell (hESC) lines which have been deemed suitable under European law to be supplied as starting material for the manufacture of advanced human therapies. In partnership with the depositing centres these hESC lines have been derived, stored and banked under conditions which meet the standards set out in the European Tissue and Cells Directives (EUTCD), as determined by UK regulators. The European Tissue and Cells Directives set a benchmark for the standards that must be met when carrying out any activity involving tissues and cells for human applications. This leads to the name ‘EUTCD-Grade’ which the UKSCB uses to refer to these stem cell lines.
For more information on the UKSCB EUTCD-Grade stem cell lines or how to obtain them visit our website (www.nibsc.org/ukstemcellbank) or email [email protected]
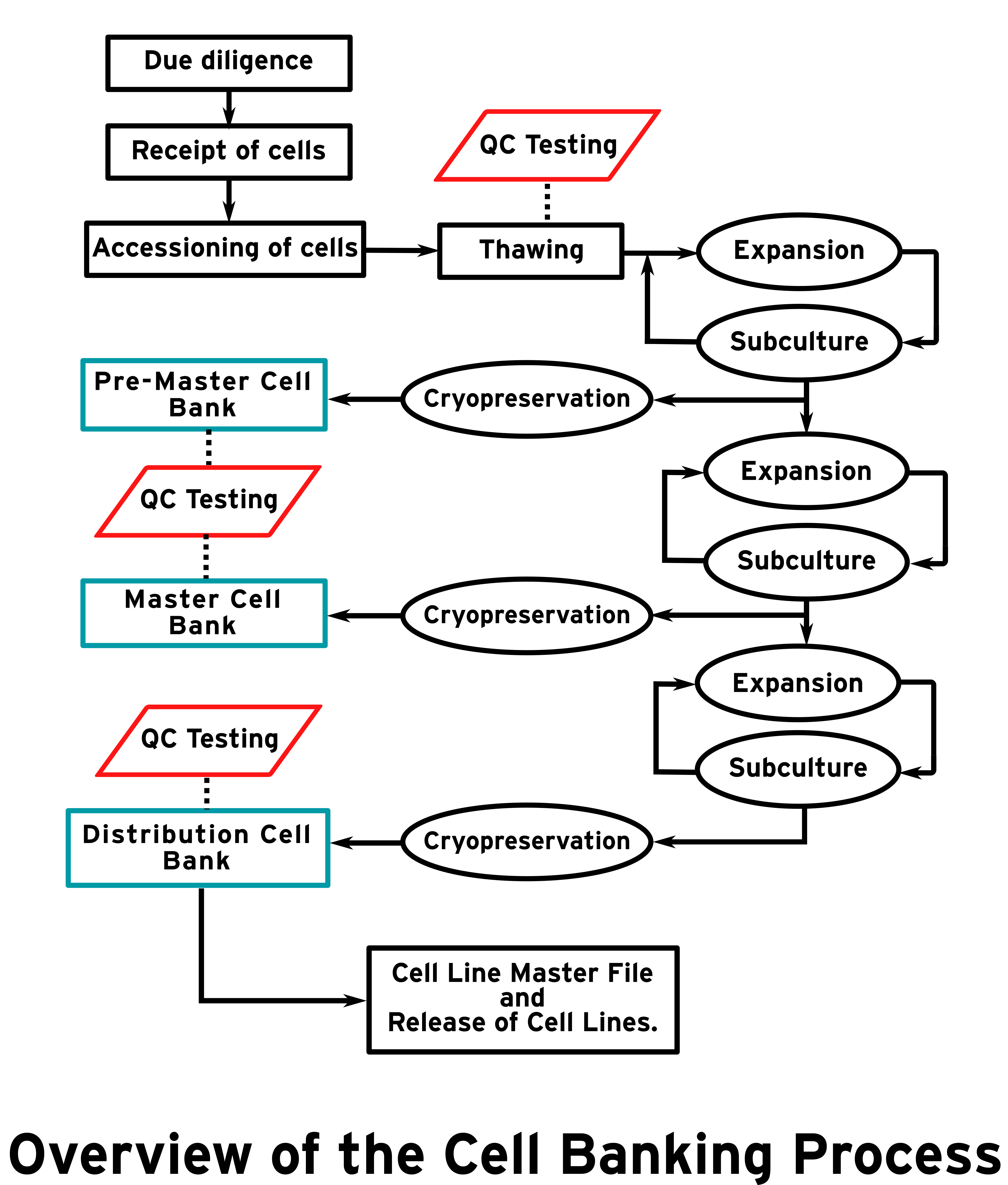
Licensed by UK regulators, the Human Tissue Authority (HTA), the UKSCB ensures that the EUTCD-Grade cell lines meet the requirements of the EU directive through a thorough and rigorous process. The process can be divided into two main sections. Firstly, what is customarily known as due diligence seeks to determine if the cell line to be deposited can be classified as EUTCD-Grade. If deemed appropriate the cell line can be taken forward into the banking process, where it is processed for distribution. Below we will explore what this means for the UKSCB and how we achieve this.
1. Due diligence — Sound Ethics and Traceability.
Before a cell line is accepted into the bank it must undergo a rigours series of checks to ensure compliance with regulation. Known as due diligence this process scrutinises the cell lines history from staff training records to adequate change controls. Two integral parts of the due diligence process are ensuring good ethical practice and full traceability has been adhered to.
EU and UK regulation necessitates the use of best ethical practice when using hESC lines and this is no different for EUTCD-Grade cell lines. To underpin this process, the UK steering committee (consisting of academics, legal experts, and government body representatives) undertakes an ethical review of all hESC lines which request to be deposited at the UKSCB. All hESC lines supplied by the UKSCB are assured to have met the best UK and EU ethical practice.
From derivation to supply the cell line material must be fully traceable. In adherence with best ethical practice after derivation the donated material is assigned a unique identifier number to ensure anonymity of the donor. A separate reference number is used during ethical review by the UK steering committee before a final accession number is assigned at the end of the due diligence process following confirmation that the cell line is fit to be classified as EUTCD-Grade. This process in combination with the UKSCBs ISO 9001 based QC systems allows anonymity and traceability throughout the process. This is critical for any cell line to be used for human application.
2. Banking EUTCD-Grade Cell Lines
Once a cell line has successfully undergone due diligence and has confirmed status as EUTCD-Grade, the banking and production process can begin. A series of expansion and subculture is undertaken to build firstly a pre-master cell bank, followed by a master cell bank, before finally creating a distribution cell bank. Key points to note about the EUTCD-Grade cell lines:
All banking is undertaken in the UKSCB’s clean rooms which meet the requirements of the European GMP standards set out in the EU Rules and Guidance for Pharmaceutical Manufacturers and Distributors.
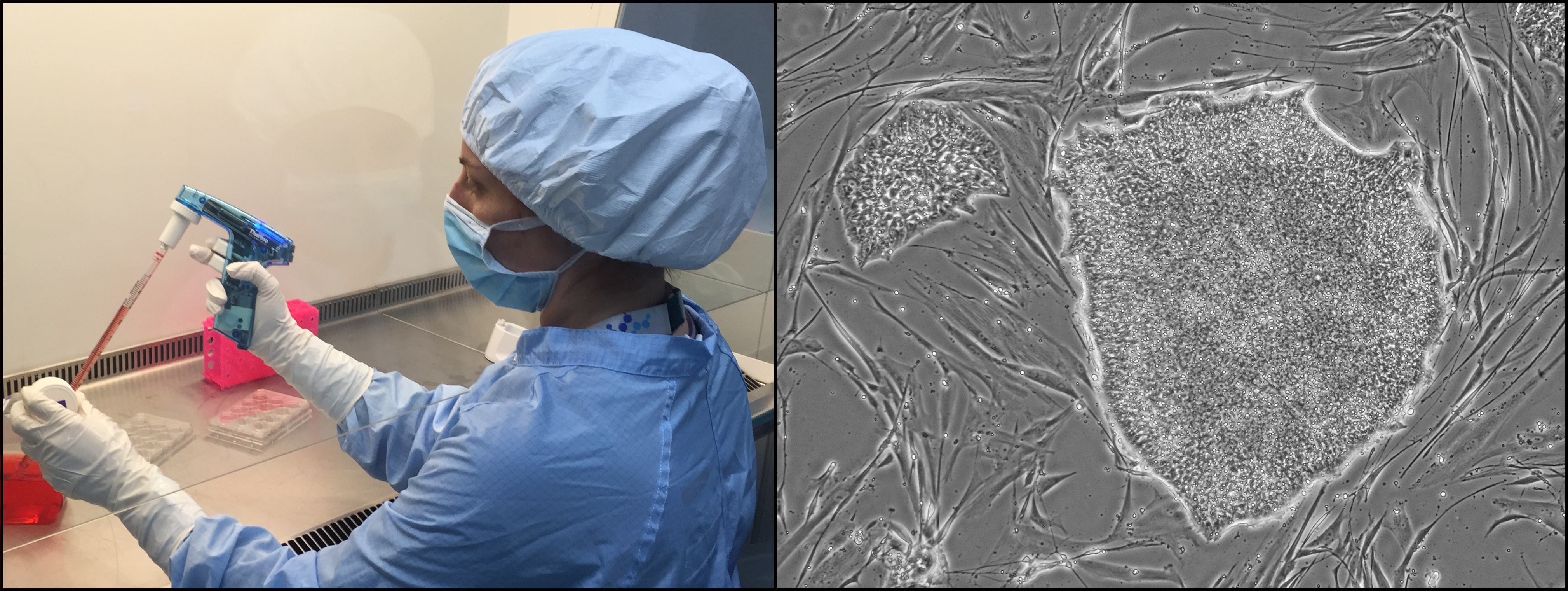
Mandatory QC testing is undertaken at the pre-master cell bank, master cell bank and distribution cell bank stages. This includes viability, sterility, in-process finger dabs, settle plates, mycoplasma, viral PCR, DNA profiling, G-band karyotyping, “Stemness” markers, differentiation and homogeneity. This data, and more, is available with each EUTCD-Grade cell line.
It is essential to note that all cell lines presented by the UKSCB as EUTCD-Grade are provided as starting materials only and their use by the user will still require careful review including a risk assessment of the cell line’s suitability for the user’s intended purpose together with an appropriate programme of risk management and risk reduction.