Next generation neural microphysiological systems: advances, applications, challenges and opportunities
In this editorial, William T Daly (University of Wisconsin Madison) presents an overview of his group’s research into the development of engineered neural models.
William T Daly1
1Managing Director / Assistant Scientist, Human Models for Analysis of Pathway (HMAP) Center, University of Wisconsin Madison
It is an exciting time to be a part of the world of stem cell engineering, biomaterial science and regenerative medicine. In particular, the ability to both understand and study the human brain has significantly increased in recent years with a rapid increase in technologies available to study both the developing prenatal, postnatal and developed adult brain and their associated neurodevelopmental and neurodegenerative disorders. These disorders are estimated to cost upwards of US$1.4 trillion in the US alone and with an increasing ageing population these costs are estimated to double by 2030.
Our research is focused on creating the next generation of human neural microphysiological systems (neural MPSs). We are focused on two categories: 1) the use of pluripotent stem cells (PSCs) and synthetic materials to create engineered ‘mini brains’ (brain organoids) with an integrated vasculature. The other is the use of microfluidics technologies that capture the minimal essential units to model an aspect of the developed or developing brain (e.g. formation or disruption of the blood brain barrier).
“It is an exciting time to be a part of the world of stem cell engineering, biomaterial science and regenerative medicine”
Neural MPSs, reviewed extensively by our colleagues (1, 2), are part of the portfolio of technologies being generated at the Human Models for Analysis Pathways (HMAPs) Center at the University of Wisconsin Madison (WI, USA). In general, organ-on-a-chip devices, MPSs, and 3D microtissues, have seen increasing prevalence in recent years, with distinct national and international programs focused on their advances e.g. the U.S. EPA Science to Achieve Results (STAR) centers, the NIH Tissue Chip Program, the NIH Tissue Chip Testing Centers. MPSs for multiple organs have now become key products of a rapidly evolving start-up community worldwide, such as CN Bio (UK), StemPharm (WI, USA), Mimetas (the Netherlands), Organovo (CA, USA) and InSphero (Switzerland), with applications in drug efficacy and safety, investigational toxicology and risk assessment of environment compounds by regulatory bodies, such as FDA and Environmental Protection Agency, and pharmaceutical companies, such as Astrazeneca (Cambridge, UK), Genentech (CA, USA), Novartis (Switzerland), Eli Lilly (UK) and GlaxoSmithKline (UK).
My research is focused on the development of neural MPSs. The complex multicellular architecture of the human central nervous system was, previously, largely considered inaccessible due to the limited availability of human tissues, need for specialized imaging to study architecture and relied on animal models that do not accurately represent the complexity of the human brain. For example, a mouse brain does present the natural folds of the human cerebral cortex or the shear complexity and number of neurons present in the human brain.
“an increased prevalence of neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease) requires better tools to understand the origins and test potential treatments”
PSCs combined with the use of biomaterials, microfluidics and tissue engineering have opened up the ability to explore molecular, cellular and tissue level architecture of the CNS. Cerebral organoid technologies pioneered by Madeline Lancaster and coworkers in 2013 (3, 4) and the group of Eiraku Sasai 5-7 have laid the groundwork for a new era of human brain research. Pluripotent stem cells from either adult induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) can be assembled into a 3D brain model through the use of defined culture methods.
These technologies have rapidly evolved and commercially kits are now available to make cerebral organoids in any lab. Using these technologies human brain characteristics such as the formation of human cortical layers and folds, defined brain regions, such as the cerebellum, striatum and hypothalamus, have been recreated in a dish (8-12). Manufacturing methods have advanced from static culture and spinning flasks to self-contained mini bioreactor systems (11) that allowed organoids to be mass produced for downstream processes such as drug discovery, drug efficacy and safety, toxin screening, and disease modelling.
In the HMAPs Center, a US EPA funded center, we are focused on the use of MPSs for vascular, neural, liver and cancer applications. My research as part of the center is focused on neural MPSs for studying neural development, understanding and treating neurodevelopmental and neurodegenerative disorders, and screening potential neurotoxins. The increased prevalence in neurodevelopmental disorders, such as autism spectrum disorders, in recent years (1 in 68 versus 1 in 2000s in 1980) (13) has highlighted the need for better tools to understand the complex etiology of diseases which are not only genetic but can also be attributed to environmental causes.
Get more from our spotlight on organoids:
- Learn more about the challenges in developing organoids and resources available to overcome them
- Read how combining microfluidics and 3D culture has created a novel microfluidic platform for cancer research
- Watch a Maryland Stem Cell Research Fund expert discuss the organoid ‘revolution’
- Use organoids to identify new therapeutics
Similarly, an increased prevalence of neurodegenerative disorders (Alzheimer’s disease, Parkinson’s disease) requires better tools to understand the origins and test potential treatments. Neural MPSs are the perfect tool for these applications. However, the application of MPSs for CNS applications despite all this promise do not come without their challenges. So what are these challenges?
For neural MPSs, one is overcoming the high variability in the generation of these self-assembling tissues, or organoids, and identifying the correct cellular components and configuration to capture the required human biology within the device. Once established, validating the human biology represented in the device is essential. Finally, balancing the level of complexity of the model, so that it can be directly translated and reproduced to pharmaceutical and industrial workflows, is key. The same challenges are common to all 3D organ models and represent a few of the challenges that have been reviewed extensively in (14). Recently, the National Institute of Health, established Tissue Chip Testing Centers which are dedicated to validating these technologies.
In my research, as part of the HMAP Center and the lab of Prof. William Murphy, we are addressing these challenges by: 1) increasing the reproducibility of 3D cortical organoid production using defined synthetic materials and highly purified cell populations; 2) integrating a defined vasculature to cortical organoid culture, an often overlooked and essential biological component; 3) validating the models using clinical biomarkers; 4) integrating organoid culture with microfluidic technologies; and 5) applying the neural MPSs in disease modelling, and environmental toxin screening.
Our lab, among others, have recently laid the groundwork for increasing reproducibility and establishing the importance of integrating vasculature in neural MPSs. One aspect we look at is the materials in which the organoids are made in. In particular, organoids, are cultured in Matrigel, an extracellular matrix derived from mouse tumors, that is intrinsically variable, complex with greater than 1500 proteins which vary substantially from batch to batch and result in poor reproducibility in organoid culture. In Nguyen*, Daly* et al. (*shared first authorship) we highlight the advantages of our synthetic alternative to Matrigel for vascular assembly and stem expansion and integration (15). These materials form the basis for increasing the reproducibility and vascularization of our neural MPSs.
“Matrigel…is intrinsically variable, complex with greater than 1500 proteins which vary substantially from batch to batch and result in poor reproducibility in organoid culture”
In Schwartz et al. (16) and Barry et al. (17) our lab has demonstrated synthetic hydrogels that have been designed to fully integrate stem cell derived vascular, neural and microglial cells. The other source of variability in organoid culture is the utilization of cells from different batches, differentiations, passages, commercial sources with undefined purities. To counter this all our MPSs are generated from highly pure (>90%) cultures of vascular, microglial and neural stem cell derived populations which are differentiated from the same parent stem cell lines using chemically defined differentiation methods. The combination of highly pure defined cell types, a defined material surface and a defined workflow has allowed us to reproducibly create engineered cerebral organoids with minimal variation (17). These models are currently undergoing evaluation at the NIH Tissue Chip Testing Centers.
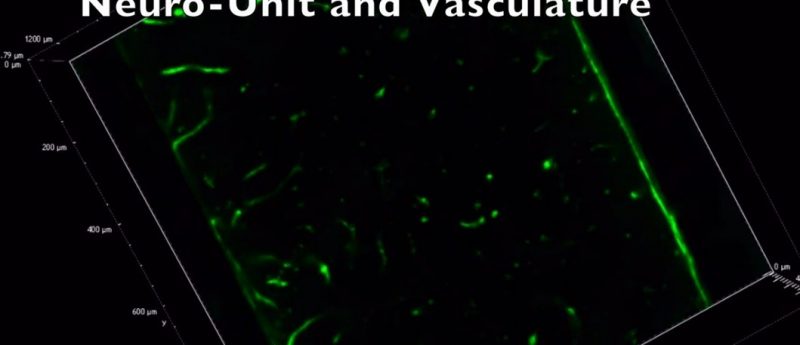
Integrating vasculature into neural MPSs is of high importance. The brain is highly vascularized with some of the highest biological energy demands in the entire human body. The brain is only initially avascular for the first 5 weeks of human brain development, but as the brain expands its depends on the infiltration of blood vessels, at 6—8 weeks, into the cortical layers to provide sufficient nutrients to the brain for its ever increasing neurological demands and for removal of toxic accumulations from cellular processes. Vasculature is therefore essential to model the brain’s natural environment and is largely overlooked in the formation of neural MPSs.
Our neural MPSs have been engineered to include a defined vasculature while maintaining distinct cortical layers and organization within the MPSs (unpublished data, to be published later this year). The vascularized neural MPSs have been integrated with a custom made microfluidic device to attempt to introduce simulated fluid flow through our engineered vessels. These combined devices are being used screen for development neurotoxins that may disrupt blood brain barrier formation or result in neurological abnormalities. For the purpose of disease modelling and validation of the biology of our neural MPSs we have taken induced pluripotent stem cells, which have been sourced from MeCP2 spectrum disorder patients, in collaboration with University of California, San Diego (CA, USA), and created the same pure cell populations to create neural MPSs that capture the phenotypical of MeCP2 spectrum disorders.
Our aim here is to correlate our findings with clinical/preclinical biomarkers to validate our model, confirm our ability to model different disease states to test the efficacy of potential drug candidates in vitro. We are looking forward publishing these studies later this year.
References
1. Kelava I, Lancaster MA. Stem Cell Models of Human Brain Development. Cell Stem Cell. 18(6):736-48 (2016).
2. Ibrahim M, Richardson MK. Beyond organoids: In vitro vasculogenesis and angiogenesis using cells from mammals and zebrafish. Reproductive Toxicol. 73:292-311 (2017).
3. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME et al. Cerebral organoids model human brain development and microcephaly. Nature. 501(7467):373-379 (2013).
4. Lancaster MA, Knoblich JA. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 9(10):2329-40 (2014).
5. Eiraku M, Sasai Y. Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr. Opin. Neurobiol. 22(5):768-77 (2012).
6. Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. U.S.A. 110(50):20284-9 (2013).
7. Nasu M, Takata N, Danjo T, Sakaguchi H, Kadoshima T, Futaki S et al. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PloS one. 7(12):e53024 (2012).
8. Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M et al. Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell. 20(3):385-396 (2017).
9. Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10(4):537-50 (2015).
10. Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Goke J et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell.19(2):248-57 (2016).
11. Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 165(5):1238-54 (2016).
12. Pasca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods. 12(7):671-8 (2015).
13. Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years -Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morbidity and mortality weekly report Surveillance summaries (Washington, DC: 2002). 65(3):1-23 (2016).
14. Marx U, Andersson TB, Bahinski A, Beilmann M, Beken S, Cassee FR et al. Biology-inspired microphysiological system approaches to solve the prediction dilemma of substance testing. Altex. 33(3):272-321 (2016)
15. Nguyen EH, Daly WT, Le NNT, Farnoodian M, Belair DG, Schwartz MP et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng. 1:0096 (2017).
16. Schwartz MP, Hou Z, Propson NE, Zhang J, Engstrom CJ, Costa VS et al. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc. Natl. Acad. Sci. U.S.A. 112(40):12516-21 (2015).
17. Barry C, Schmitz MT, Propson NE, Hou Z, Zhang J, Nguyen BK et al. Uniform neural tissue models produced on synthetic hydrogels using standard culture techniques. Exp. Biol. Med. 242(17):1679-89 (2017).





