Counting keywords is not compiling clinical trials
In this excerpt, Cell Trials Data discusses whether a simple keyword search is an accurate way to find MSC trials on ClinicalTrials.gov.
This post is an excerpt from an article posted on CellTrials.org. Read the full article.
The number of mesenchymal stem cell (MSC) trials registered each year has increased 50% in the past five years (2013-2017) from about 100 to 150 trials per year. During the past five years the fraction of MSC trials that rely on perinatal sources has risen from about 20% to 30%. In 2016 and 2017, trials with perinatal MSCs outnumbered bone marrow- derived MSCs or adipose tissue-derived MSCs. The perinatal sources include umbilical cord blood, umbilical cord tissue and various types of placenta tissue.
We wanted to know whether searches on the keyword “mesenchymal” are an accurate way to find MSC trials. Although the word “mesenchymal” is pretty unique, that fact that it appears somewhere in the description of a clinical trial does not guarantee that the trial relies on the action of MSCs.
We tested trials in ClinicalTrials.gov that contained the keyword “mesenchymal” and were first posted between 1 January 2011 and 31 December 2017. We found 767 trials and after examining each one we concluded that 630 trials actually rely on the action of mesenchymal cells, while the remaining 137 “mesenchymal” trials were false positives. The false positives included: trials in which the MSC are not fully isolated from their source, trials that rely on the action of cytokines released by MSCs but not the cells themselves, studies of MSC isolation procedures and miscellaneous trials involving cell biology or drug testing etc.
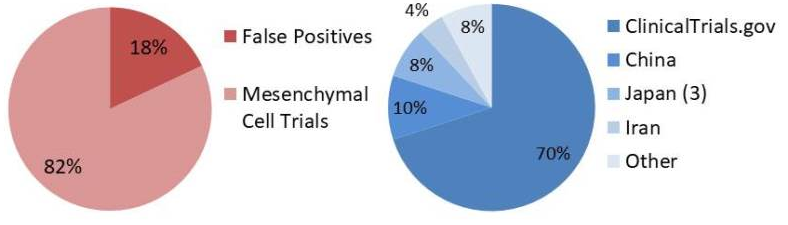
We find that only 82% of the trials returned by the keyword “mesenchymal” are actually trials that rely on the action of mesenchymal cells. Any automated tools that collect MSC trials from ClinicalTrials.gov by keyword search will only find 70% of the world’s MSC trials and will only have 82% accuracy in the trials retrieved from ClinicalTrials.gov.
This post is an excerpt from an article posted on CellTrials.org. Read the full article.
The Cell Trials Data Team consists of Frances Verter PhD, Alexey Bersenev MD PhD, and Pedro Silva Couto MSc. Meet the team here.