3 Ways to Verify iPS Cells Using a Single Well
Don’t choose between experimentation and characterization. This article discuss easy methods to routinely validate pluripotency without sacrificing precious pluripotent stem cells.
Pluripotent stem cell characterization is a crucial process for establishing new induced pluripotent stem (iPS) cell lines as well as for monitoring the quality of routinely cultured embryonic stem (ES) and iPS cells. It is critical because the purity of iPS cells and their capacity for differentiation directly affects the productivity and efficiency of cell expansion as well as the generation of terminally-differentiated cell types.
The Problem
Incorporating characterization as a routine step when working with ES and iPS cells can be difficult. Many methods for culturing, expanding, and differentiating cells are suboptimal and inefficient, resulting in limited quantities of cells being available for downstream experimentation. Researchers must then prioritize: should all of the stem cells be used for differentiation and downstream application or should the experiment be smaller so that some cells can be used to assess the quality and consistency of the iPS cells line? The former is most often chosen over the latter. However, as I wrote about in a previous post, maintaining the quality of iPS cell lines is an issue currently facing stem cell research and an important consideration to keep in mind when designing experiments.
A Solution
The research and development team at Bio-Techne has a poster discussing methods that make it easier for researches to have enough cells for characterization and experimentation. Specifically, single wells of a 24-well plate can be used to verify pluripotency by protein expression, immunofluorescence, and tri-lineage differentiation.
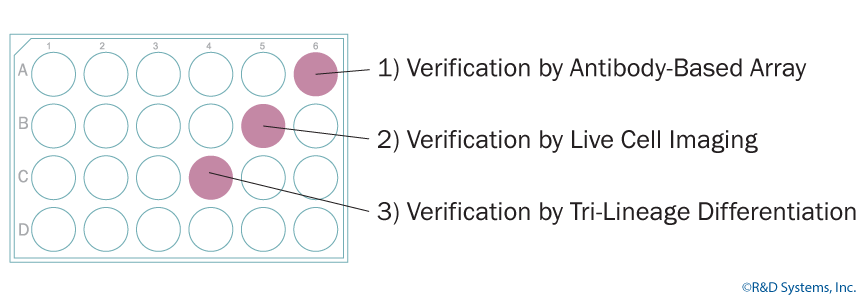
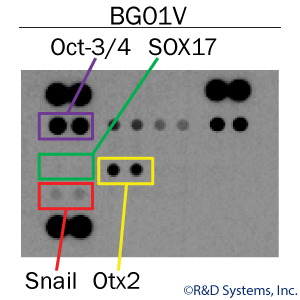
Verification by Antibody-Based Array
One well of a 24-well plate can be used to probe simultaneously for both markers of pluripotency and markers for the three primary germ layers, endoderm, mesoderm, and ectoderm. Using cell lysates from a single well of pluripotent stem cells along with the Proteome Profilerâ„¢ Pluripotent Stem Cell Array Kit, researchers can easily assess the purity of ES and iPS cell populations. In this example, human embryonic stem cell lysates were shown to express the positive pluripotency marker, Oct-3/4. While no endoderm (SOX17) and mesoderm (Snail) markers were expressed, the starting population did show low expression of Otx2, an ectoderm marker. Thus, the starting stem cell population was not completely pluripotent which could affect experiment efficiency and data reproducibility.
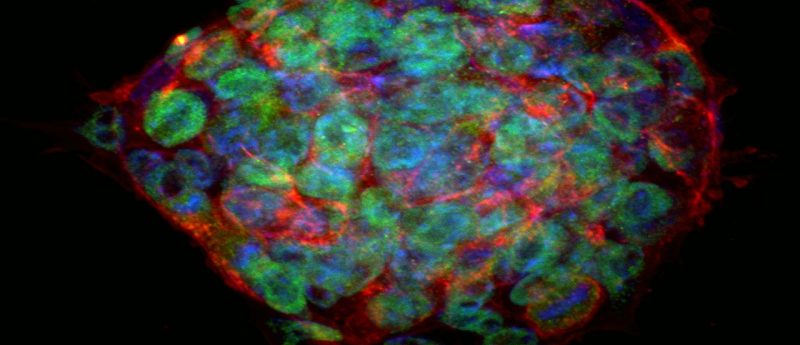
Verification by Live-cell Staining
One well of a 24-well plate can be used to screen for expression of known surface markers for ES and iPS cells. GloLIVEâ„¢ antibodies for SSEA-1, SSEA-4, TRA-1-61, and TRA-1-81 are fluorochrome-conjugated and are added directly into a well for real-time visualization of pluripotent stem cell markers. An added benefit of GloLIVE antibodies is that the stained cells retain their stemness and can continue to be used for expansion and differentiation. View the poster for data.
Verification by Tri-Lineage Differentiation
Perhaps the most critical of the three verification methods, validating the tri-lineage differentiation capacity of your stem cells, will provide you with the best information about how apt your cells are for differentiation. The Human Pluripotent Functional Identification Kit from R&D Systems provides optimized cocktails of growth factors for pushing pluripotent stem cells into endoderm, mesoderm, and ectoderm. These cocktails are added to individual wells of a 24-well plate and differentiation is complete within 5 days. Compared to the teratoma assay, this kit is a quick and standardized way to assess the pluripotency of ES and iPS cell lines.
Poster Summary and Highlights
- Easily methods for routinely validating your pluripotent stem cell populations.
- All three techniques can be performed on cells cultured and differentiated in a 24-wellplate, limiting the number of cells needed for verification and conserving cells for experimentation.
- The combination of antibody-based array, live cell imaging, and tri-lineage differentiation provides the highest level of verification for pluripotent stem cells.
- Verification with these three methods ensures the highest quality of cells providing better experimental and consistency for downstream experiments.