A love affair with the blastocyst: an interview with Janet Rossant
In this interview, Janet Rossant (The Hospital for Sick Children, ON, CA) discusses what we can learn from non-human models and why it’s important to mentor minorities in science.

Janet Rossant
Rossant trained at the Universities of Oxford and Cambridge (UK) and has been in Canada since 1977, first at Brock University and then at the Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto (both ON, Canada), from 1985 to 2005. Rossant has been recognized for her contributions to science with many awards, including the CIHR Michael Smith Prize in Health Research, Canada’s most prestigious health research award, was the first female to receive the 2015 Canada Gairdner Wightman Award and was recently awarded the L’Oréal-UNESCO For Women in Science Award. She is a Fellow of both the Royal Societies of London and Canada, and is a foreign Associate of the US National Academy of Science. Rossant was the Director of the newly formed Ontario Institute for Regenerative Medicine, Senior Scientist, Developmental & Stem Cell Biology program, The Hospital for Sick Children (SickKids), and Professor, Department of Molecular Genetics, and the Department of Obstetrics and Gynecology, University of Toronto (all ON, Canada).
Please introduce yourself and tell us about your career so far
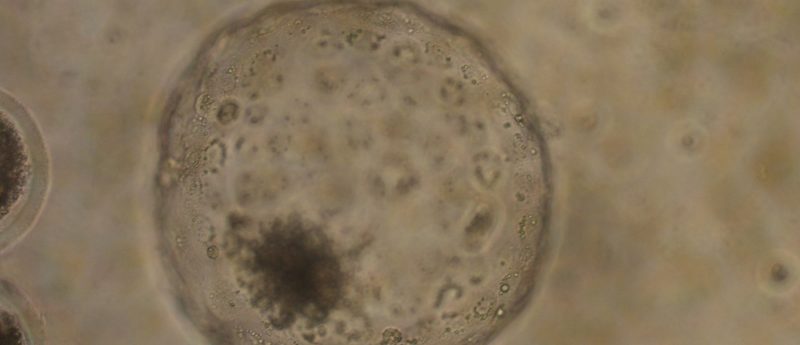
I trained in the UK with Richard Gardner who was the first person who was able to make chimeras by putting cells back into blastocysts, a technology that’s gone on to be incredibly important for studying stem cells. As a graduate student, I was very interested in the development of the early embryo and started my love affair with the blastocyst. The blastocyst is the first stage of development in mammalian systems, whether it’s a mouse or a human, where you get the first differences between cells. The outer cells, called the trophectoderm, go on to make the placenta, which the mammalian embryo needs to survive in the uterus.
“I think it’s my job to mentor all young scientists that come into the field”
I spent my early years trying to understand how those first cell lineages are set aside in the embryo. We used chimeras and other approaches to follow the fate of cells from the blastocyst into later development. That led us to show that the trophectoderm makes the placenta, with another set of cells that make the yolk sac and a minority of cells, that we now call the epiblast, making everything else; the fetus and a few things beside. These are the cells are what we now call pluripotent. In the early days, we were looking at the embryo and identifying pluripotency, and, many years later, this has gone on to be the foundation of what we now know about pluripotent stem cells because those pluripotent stem cells arise from the pluripotent cells of the embryo.
In my work over the years I’ve used pluripotent stem cells in the mouse to undertake a lot of genetic analysis by knocking out genes that are important in the development and formation of organs such as blood vessels, the heart and the placenta. There are many different kinds of genetic pathways that are involved in normal development and organ formation, and we keep coming back to blastocysts to dig for more detail on them.
“I do believe that we’re going to see cell therapies derived from stem cells”
In recent years, we’ve used the new tool of live imaging, fluorescently tagging proteins for single-cell analysis. We’ve continue to go back to look at the dynamics of genetic control and cell lineages of these processes. In the last year we’ve been using CRISPR-Cas9 to rapidly generate fluorescently tagged genes that mark all the different lineages. We’re just beginning to put together a sort of dynamic process to study blastocyst development, and watch multiple gene products change their distribution and function during the early stages of development.
What can we learn from mice that can be applied to humans?
Whether we’re looking at early development, organ formation or disease pathways, humans and mice are very similar. If we had not studied the mouse blastocyst and didn’t know about pluripotent stem cells in the mouse, we would never be doing pluripotent stem cell biology in humans. Although many of the fundamental genes are the same, there are differences that we have to be aware of; I think these are important because they also illustrate the sort of variation that one can have in genetic pathways and still end up with normal development.
Therefore, it is very important to carry out research side by side in human and mouse, whether it’s embryonic development or stem cell biology. I’ve said several times in the press that I think it is important to study those differences if we want to be able to understand pregnancy problems in humans or early developmental defects and if we want to make better stem cells. Although the fundamentals are the same, the details do matter, so we need to do more work with human embryos, embryonic stem cells and pluripotent stem cells, and compare and contrast with mouse.
You studied in the UK but now live and work in Canada. Why is this?
Canada is a great place to be living and doing science. I think regenerative medicine and stem cell research is a strength in Toronto in particular, and across Canada. That goes back to the legacy of Till and McCulloch who developed the concept of stem cells. That legacy has continued over the years as more people have worked with them and been trained so we have a very strong basic stem cell biology background.
We also have a very collaborative environment in Canada. We have built networks that bring together stem cell biologists, bioengineers, clinicians and ethicists all together to look at the whole ecosystem of stem cell biology and, increasingly, take that forward to the clinic and commercial applications. The Canadian Stem Cell Network, which has been running for 16 years, has been a key part of this and, from that, we’ve been able to build local networks such as the Ontario Institute for Regenerative Medicine. They also give some foundational money to build those networks so we can start seeing how we can take the discoveries that we’ve made from basic biology into the clinic.
It has been said that regenerative medicine hasn’t lived up to its promise; do you agree?
I think that you have to be very careful of balancing hype versus hope. In the beginning with pluripotent stem cells there was a lot of early hype. These are cells that can make every cell type in the body and if we can control that, and control the safety, we do have an amazing potential.
It does take time and there are lots of steps along the way where things can go wrong. I think that there was an overhyping with the field at the beginning that was largely driven by politics. The need was felt in many jurisdictions, particularly the US, to really insist on the importance of this approach because of the jurisdictions that were banning human embryonic stem cell research. I think now we’re over that hype — we’re in the middle phase — and we are seeing real steps. I would note that there are clinical trials going on for macular degeneration, we’re seeing the first clinical trials going forward with pluripotent stem cell-derived cells for diabetes and very shortly I think we’ll see trials for Parkinson’s.
“As a graduate student, I…started my love affair with the blastocyst”
Everybody expects things to move much faster but if we compare it with other areas, for example, drug development, this is no faster or slower. I think the future is not all about pluripotent stem cells though; it’s actually very exciting to see other kinds of cell therapies coming forward, such as immunotherapies with CAR-T cells and so on. New tools of gene editing are also going to mean that we’re going to see new ways of using adult stem cells to correct diseases.
You recently received the L’Oreal-UNESCO Women in Science Award. As an influential female scientist, why is it important to mentor women in science?
I think it’s my job to mentor all young scientists that come into the field. In any area of expertise, including regenerative medicine, we need to be able to draw on diversity, wherever there’s brain power. In science, women are still not fully represented. It’s important to be a role model for women and show that it’s possible to have a career, a family and move forward; people should not be put off science. Often, at school, women will be dissuaded from going towards a scientific career, being told “it’s too difficult” or “you can’t have a family”; this is a totally outdated concept.
“Canada is a great place to be living and doing science”
However, the problem is not always in the bottom level. In many parts of the world, education for girls and women is very difficult to access. In countries like ours, where access to the education is not the issue, we’re seeing women coming into science at the undergraduate and graduate level. There’s still a lack of women in the physical sciences and computer sciences but life science is well represented. As you go up the ladder in the academic world, women are not as well represented. If you think about it as a pyramid, the base of the pyramid has stretched out but not enough women are moving up. The best way to make that happen is to have strong role models at higher levels to encourage women to move forward.
What are your hopes for the future of regenerative medicine?
It’s very exciting actually working on embryo development then working on stem cells. My lab works with human embryonic stem cells and induced pluripotent stem cells (iPSCs) in a big group studying cystic fibrosis. We try to make lung and other cell types using iPSCs so we can monitor the disease in in the petri dish and give individual kids the right treatments. I’ve spent a lot of time, over many years, writing grants, saying “what we study here will be important” and “we’ll be able to capture this and use that”. Now, we can use the tools of stem cell biology to study and model disease, and develop new drugs. I do believe that we’re going to see cell therapies derived from stem cells.