Adopting automation for the manufacture of cell products — considerations and best practices

In this community post, Jelena Ochs, Fraunhofer Institute for Production Technology, discusses the challenges of utilizing automated solutions in biological processes.
Research on cell therapies continues to deliver promising results for regenerative applications. Now that CAR-T therapies have been approved as a combined cell and gene therapy [1], it may seem as if the access to advanced therapies are finally within reach. In reality, however, the industry still faces a number of challenges related to the manufacture of the cell products [2].
While the traditional bioprocess industry applies scalable solutions for production, the situation in the advanced therapy industry is more challenging, due to the inherent complexity of the cell products. With therapies that are often autologous and input material from donors that is subject to a strong batch-to-batch variation, an industry of scale is less feasible. This further increases costs of the already expensive manufacturing, which requires skilled labor and considerable efforts to ensure safe and efficacious products.
For this reason, the industry is moving towards automation of closed, parallelized processes. The promise of automation is to reduce the manual labor and thus risks of human errors as well as to increase reproducibility. Additional values lie in the fact that processes can be monitored and documented more comprehensively.
What is automation?
The term “automation” is thereby not exactly defined. It is used to describe devices that automate individual steps, such as bioreactors or pipetting robots. In recent years, automated systems are becoming increasingly functionally integrated, so that several steps can be carried out on the same device. Fully automated, robot-assisted systems that require little to no human intervention are currently still uncommon. However, as the consortium of the EU project “AUTOSTEM” has recently demonstrated, such concepts are feasible and suited to shape the production of therapeutic cell products in the future [3, 4].

In order to ensure a seamless transition into automation, it is important to think about automation early — in the best case when the first protocols for generation of the cell products are developed. Some manufacturers believe that they remain more flexible and save costs when they switch to automation at a rather late stage – and then become stuck with protocols that are difficult to automate. Instead, processes should be designed in a way that they can be transferred easily later.
One example is the cultivation of cells in T-bottles, a process that is common in research laboratories, but is not practicable for automation. By switching the cultivation systems to spinner flasks or small-scale bioreactors on the other hand, processes can be translated more easily to scalable and automated bioreactors at a later stage. Difficult to automate are also elaborate handling steps and processing decisions that rely on experience or on non-objective assessment of the operator.
Streamlining adoption
Manufacturers that make sure that their processes are in general automatable, will save time and effort when they are translated onto automated equipment. In any case however, it has to be ensured that the product still remains the same when automation is increased or the production strategy is changed altogether. Therefore, manufacturer have to ensure they know the product and understand the processes. In the context of the Quality by Design approach, this means understanding the critical quality attributes (CQA) of the product and the critical process parameter (CPP) that influence the quality [5].
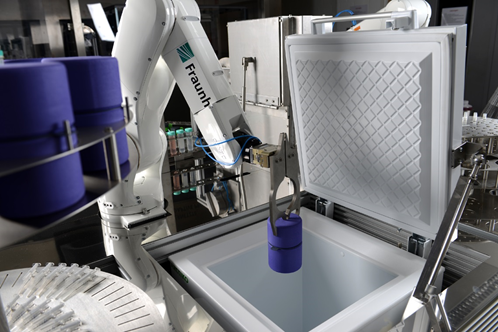
In order to monitor and control these process parameter and cell product throughout the process, it makes sense to invest in suitable process analytical technology. Bioreactors typically are equipped with sensors that measure dissolved oxygen or pH. More sophisticated technologies to measure cell density or metabolites in the medium are currently being developed to provide more information in the future. In the context of automation, the possibilities are further increasing. Whether it is automated microscopy in combination with image processing algorithms [6] or integration of other analytical devices such as cell counters, automation allows to integrate quality control and product characterization inline during the process.
On this basis, not only comprehensive reports on the manufacturing process can be generated, but it is also possible to use the data inline for feedback control and objective decision. From here on, the leap to full automation is not far away: by interconnecting devices on the software level, data can be used as a basis for control loops automated decision trees [7]. More elaborate on the other hand is to combine this with a physical interconnection, which can be achieved by integrating robotic arms. This concept has already been applied for parallelized, small-scale production of induced pluripotent stem cells [8] and with AUTOSTEM more recently also for bioreactor-based production of mesenchymal stem cells [3, 4].
Ensuring compliance of automated processes
Since cell based therapeutics fall under the category of advanced therapy medicinal products (ATMP), it has to be ensured that the automated production is in compliant with the regulatory framework. In the case of automated production, manufacturers have to ensure that they do not only comply with Good Manufacturing Practice (GMP) but that their computerized systems are also validated according to good automated manufacturing practice (GAMP). This should be taken into account when selecting suppliers for automated devices. In addition, Annex 11 of the EU-GMP guideline and the 21 CFR Part 11 published by the FDA provide the regulatory framework for electronic records.
In many cases, the development and implementation of new automation solutions is a task for interdisciplinary teams consisting of biotechnologists, mechanical and automation engineers as well as computer scientists. For manufacturers, this does not necessarily mean that they have to have all these experts in-house – but they should be open to interdisciplinary cooperation. This way, they can also benefit from new approaches, such as emerging technologies from industry 4.0 or artificial intelligence [9]. Currently, these technologies are still relatively new for the industry, but they pose substantial potential to make processes more robust, efficient and reduce costs. In the end, these technologies can deliver valuable contributions towards the joint goal of making cell therapy more accessible and affordable.
Financial Disclosure / Acknowledgements
Fraunhofer IPT has been funded for the AUTOSTEM project by the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 667932. Additional funding has been provided for the project “StemCellFactory III”, funded by the European Regional Development Fund (ERDF) under grant number EFRE-0800972.
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
[1] FDA approves Gilead cancer gene therapy; price set at $373,000 – Reuters. [Online] Available: https://uk.reuters.com/article…. Accessed on: Jul. 22 2019.
[2] Heathman TR1, Nienow AW, McCall MJ, Coopman K, Kara B, Hewitt CJ. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen. Med. vol. 10, no. 1, pp. 49—64 (2015).
[3] Ochs J, Barry F, Schmitt F, Murphy JM. Advances in automation for the production of clinical-grade mesenchymal stromal cells: The AUTOSTEM robotic platform. Cell Gene Therapy Insights. vol. 3, no. 8, pp. 739—748 (2017).
[4] AUTOSTEM — stem cell manufacture. [Online] Available: http://www.autostem2020.eu/. Accessed on: Jul. 22 2019.
[5] Lipsitz YY, Timmins NE, Zandstra PW. Quality cell therapy manufacturing by design. Nat. Biotech. vol. 34, no. 4, pp. 393—400 (2016).
[6] Schenk FW, Kulik M, Schmitt R. Metrology-based quality and process control in automated stem cell production. tm – Technisches Messen. vol. 82, no. 6 (2015).
[7] Jung S, Ochs J, Kulik M, König N, Schmitt RH. Highly modular and generic control software for adaptive cell processing on automated production platforms. Procedia CIRP, vol. 72, pp. 1245—1250 (2018).
[8] Marx U, Schenk F, Behrens J et al. Automatic Production of Induced Pluripotent Stem Cells. Procedia CIRP. vol. 5, pp. 2—6 (2013).
[9] Kulik M, Ochs J, König N, Schmitt R. Automation in the context of stem cell production — where are we heading with Industry 4.0? Cell Gene Therapy Insights, vol. 2, no. 4, pp. 499—506 (2016).