De-risk and industrialize cell and gene therapy — a process development roadmap
Lonza (Basel, Switzerland) explains how they can assist in guiding a cell therapy from pre-clinical development to commercialization.
Transitioning from clinical-stage processes to a commercial therapy can be a complicated journey with major challenges, and many organizations will underestimate the significance of high-risk gaps existing in the manufacturing process.
“Good analytical methods need to be robust and they need to be in place before the process is defined,” commented Robert Schrock, Head of Bioanalytical Services for Lonza Cell and Gene Technologies Development Services (TX, USA).
Lonza, a world-renowned custom development and manufacturing organization in the field of biological science, offers a service to ease the transition of cell therapies to the clinic by harnessing their years of experience. With their teams of experts across three continents, Lonza can identify the potential issues within a manufacturing platform at an early stage in the transition, mitigating the financial fallout of correcting the process later.
According to Francesca Vitelli, Head of Virus Development (Lonza) at the time of filming, “process, analytical design and development become the gatekeeper to the success or failure of a potentially lifesaving product.”
In the market for over 20 years, Lonza also guides their clients through the complicated web of regulations, utilizing their in-depth knowledge of the regulatory frameworks around the world to lead a client’s cellular therapy from Investigational New Drug application to commercialization. To achieve this, Lonza draws upon 140 dedicated technical experts and the highest quality GMP-compliant techniques to produce a holistic approach to prepare a cellular therapy for the market, working with the client at every turn.
Vitelli also suggested that “success in process development really is a question of balancing the rigor of the scientific process with the creativity required to find innovative solutions.”
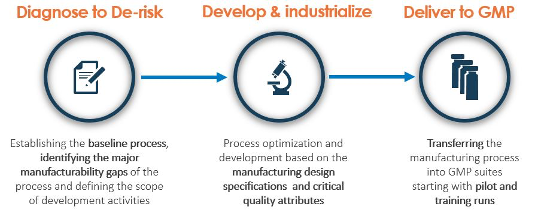
Lonza takes a step-by-step approach towards the development of manufacturing processes for cell and gene therapy applications. By establishing a deep understanding of their customers’ needs, considering their timeline and phase of application, Lonza has produced a phase appropriate process development exercise. They also collaborate very closely with their customers and involve them in each stage or phase of the project.
Offering collaborative workshops and even opportunities to exchange experts for training, Lonza aims to become an extension of their client’s team and bring the therapy to market as quickly and efficiently as possible.
If you are interested in further discussing your process development needs, you can find more information at Lonza Pharma and Biotech or fill out the form below.
**Please note, after successfully submitting the form you will be returned to the top of this article.