Developing 3D bioprinted, vascularized human skin for improved wound healing
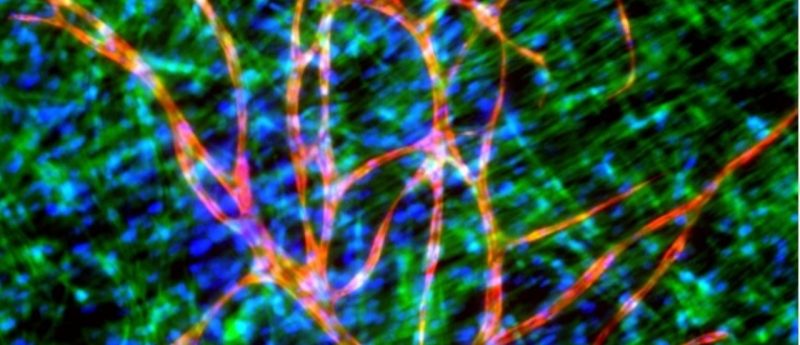
Providing a vascular network to promote survival of cells for full-thickness wounds is crucial for proper perfusion and survival of skin-grafted tissues.
Tânia Baltazar1 & Pankaj Karande2
1PhD student, Department of Chemical and Biological Engineering, Rensselaer Polytechnic Institute, Troy, NY, USA
2Associate Professor, Department of Chemical and Biological Engineering, Rensselaer Polytechnic Institute, Troy, NY, USA
Treatment of chronic skin wounds remains a significant clinical challenge. Autologous split-thickness skin grafts represent the ‘gold standard of care’, but are limited by the pain and discomfort associated with harvesting skin from donor sites, limited availability of donor sites, and the need for multiple surgeries.
To overcome this challenge, many tissue-engineered skin substitutes using biological and artificial matrices and/or allogeneic cells have been developed, and are beneficial in conditions when urgent care is needed. However, the use of foreign or synthetic materials to cover the wounded area is only a temporary solution, and suboptimal engraftment often leads to unsatisfactory clinical results [1].
When the clinical situation allows for a delayed reconstruction of the defects such as chronic wounds, tissue engineered skin from autologous cells is the ideal choice. Nevertheless, there are several critical hurdles that must be overcome. Current artificial skin grafts do not include dermal vascular networks important for graft survival and integration with the native tissue. Poor vascularization after implantation often compromises the survival of skin grafts.
Providing a vascular network to promote survival of cells for full-thickness wounds is crucial for proper perfusion and survival of the grafted tissue, especially when the tissue is thick and its survival cannot be supported by local diffusion of oxygen and nutrients [2]. Therefore, there is an urgent and critical need to address these gaps in order to develop better wound care products for chronic wound treatment.
At the Rensselaer Polytechnic Institute, we are addressing these gaps using a 3D bioprinting platform to fabricate a vascularized skin equivalent constituted entirely of primary cells (keratinocytes, endothelial cells and dermal fibroblasts). Preliminary data in our lab demonstrated that 3D printed skin tissue is morphologically representative of in vivo human skin tissue. In addition, we are developing printed endothelial networks that are able to generate interconnected tubular structures with stable lumens within a 3D collagen matrix that persist beyond 50 days.
We anticipate that this new strategy for tissue vascularization via 3D cell printing technology will dramatically improve skin graft survival in vivo and shorten the time of graft incorporation with the host skin. This platform will allow for complete automation of vascularized skin fabrication in a high-throughput manner constituting the first step towards the development of a full thickness skin equivalent generated entirely with 3D printing technology.
Furthermore, 3D printed vascularized skin can also serve as a valuable in vitro human model as an alternative method to animal testing. Evaluation of permeability and toxicity responses of topical and transdermal agents during the preliminary stages of drug discovery and formulation development will not only improve the confidence in the experimental results before initiating human clinical trials, but will also significantly decrease the cost of drug development by pharmaceutical companies.
References
- Böttcher-Haberzeth S, Biedermann T, Reichmann E. Tissue engineering of skin. Burns 36 (4), 450—460 (2010).
- Frueh, FS, Menger MD, Lindenblatt N, Giovanoli P, Laschke MW. Current and emerging vascularization strategies in skin tissue engineering. Critical Rev. Biotech. 8551, 1—13 (2016).