Developing a novel biphasic barrier membrane for guided tissue regeneration
In this interview, Serkan Dikici and Betül Aldemir (University of Sheffield) discuss their project on producing scaffolds for guided tissue regeneration.
This interview is part of our focus on regenerative medicine. Visit the focus>>
Serkan Dikici* & Betul Aldemir*
Department of Materials Sciences and Engineering, The University of Sheffield, United Kingdom
*equally contributed to this work
Please introduce yourself and your institution
We are two graduate students who are undertaking our PhDs in Prof Sheila MacNeil’s and Dr Frederik Claeyssens’s group in Biomaterials and Tissue Engineering group at the University of Sheffield (UK). This group focuses on developing biomaterials and tissue engineering which will benefit patients, alongside fundamental work to develop new understanding and tools in the area of tissue engineering. A key feature of the group is that it encourages multidisciplinary interactions, bringing scientists, engineers and clinicians together to give a broad range of research expertise.
Can you explain the background to this research?
This biphasic barrier membrane research is a collaborative study which aims to combine the advantages of two ongoing projects in the group: nanofibrous drug releasing scaffolds for promoting angiogenesis and highly porous interconnected materials for bone tissue engineering. First is a nanostructured polycaprolactone (PCL) barrier membrane, which acts as a physical barrier for preventing epithelial invasion for up to 4 weeks without limiting the diffusion of waste and nutrients. This is then combined with the second biomaterial — a scaffold of polymerized high internal phase emulsion (polyHIPE) templated PCL that encourages and guides bone formation because of its highly porous and interconnected structure. This is being developed for patients who require bone grafting surgery.
What are the benefits of using a tissue scaffold in tissue regeneration?
The success of engineering tissues in vitro is a balance between three components, which are known as tissue engineering triad: biomaterials (or scaffolds), cells and growth factors. Tissue engineering scaffolds can be considered as the templates for newly forming tissues and they have vital importance for the proliferation and differentiation of cells, and engineering tissues in 3D. Successful tissue regeneration can be achieved using tissue scaffolds with appropriate mechanical and surface properties, biodegradability, macro and micro porosity to provide a better microenvironment for cell—cell and cell-material interaction. This will then lead to cell migration, proliferation and differentiation of tissues. In the case of dental bone grafts, using a barrier membrane scaffold is crucial to prevent epithelial cell invasion into the bone growing zone. This is necessary for new bone regeneration as bone is relatively slow growing tissue compared with the rapid formation of epithelial tissues in gum tissue.
What are the biphasic PCL scaffolds made from and how are they produced?
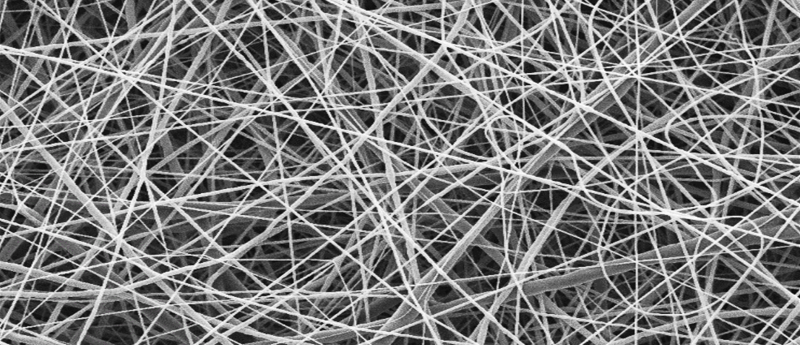
Biphasic PCL scaffold is a barrier membrane which is composed of electrospun PCL nanofibers, which act as a physical barrier for preventing epithelial invasion for up to 4 weeks without limiting the diffusion of waste and nutrients, and polyHIPE PCL that encourages and guides bone formation with its highly porous and interconnected structure. The polyHIPE PCL scaffold was manufactured via an emulsion templating technique. Briefly, methacrylate functionalized PCL (PCLMA) was synthesized and this PCLMA was then used to form a water-in-oil (w/o) emulsion. This emulsion was then cured under UV light to produce microporous scaffolds.
To produce a physical barrier, electrospinning was used to make a nanoporous membrane. PCL was dissolved in a solvent composition which can create nanofibers and then electrospun onto polyHIPE PCL scaffolds using the optimized parameters to create biphasic PCL barrier membrane. The key features are that these fibers are thin and tightly packed together so that cells cannot migrate through them.
How do the barrier membrane properties of the scaffold determine the effectiveness of the scaffold for tissue regeneration?
Bone is a relatively slow-growing tissue when compared with epithelial tissues. An epithelial invasion into the bone-growing zone will make it even slower by suppressing the growth of bone cells. That is why limiting the growth of epithelial cells is really important for the healthy regeneration of bone tissue. On the other hand, encouraging the growth of bone cells using a microporous and interconnected scaffold will accelerate the bone regeneration. The barrier membrane properties of the scaffold provide these two important functions: a limitation of epithelial invasion and promotion of the ingrowth of osteogenic cells.
What are the potential applications for the scaffold?
Biphasic PCL scaffolds can be used in various applications which require guided tissue regeneration (GTR). GTR is widely employed in periodontal regenerative medicine, and barrier membranes can be used in reconstruction of bone defects, sinus lift elevation operations, building up bone around implants and the repair of bone defects surrounding dental implants, amongst other things. Although there are plenty of application areas, the essential criteria for developing a barrier membrane are being biocompatible, being able to act as a physical barrier, allowing tissue integration, having appropriate mechanical properties, and being suitable for clinical translation.
The great thing about biphasic PCL barrier membrane is that it is possible to modify these membranes according to the intended aim of use. They can be produced in different shapes and sizes and different microstructures (different pore sizes and porosity) and mechanical properties. Being able to tune the properties of our membranes expands their field of applications.
What are the next steps in this project?
The physical barrier function and bone promoting properties of biphasic PCL membranes have already been studied in vitro. However, there are still plenty of things to optimize. Our group is currently working on two great alternatives of vascular endothelial growth factor (VEGF) to drive angiogenesis. They are inexpensive, stable and approximately 80% as potent as VEGF. Combining these small natural molecules with the barrier membrane may overcome more than one problem at the same time: a drug releasing barrier membrane scaffold which limits the epithelial invasion and promotes the growth of osteogenic cells, and at the same time, stimulates neovascularization. The next step of this project is to test the barrier membrane properties of our biphasic PCL scaffolds using in vivo models.