Introduction to the MDI Biological Laboratory’s Center for Science Entrepreneurship

New center will focus on driving innovation and entrepreneurship in the life sciences.
In March 2017, the MDI Biological Laboratory in Bar Harbor, Maine, will open its new Center for Science Entrepreneurship. The completion of the $3 million facility marks a major milestone in the history of the 119-year-old institution, reflecting its 21st century focus on pioneering new approaches to regenerative medicine and creating a global hub for biomedical innovation and entrepreneurship.
Regenerative medicine is revolutionizing 21st century health care. Scientists at the MDI Biological Laboratory are leading this revolution through their focus on identifying the innate mechanisms that allow tissues to regenerate after damage. Using this knowledge, they are developing drugs that activate these innate mechanisms in order to restore the function of tissues and organs that have been damaged or lost to disease, injury and aging.
The new center also reflects the institution’s commitment to accelerating innovation and modernizing Maine’s economy through the creation of a life sciences commercialization ecosystem to incubate and attract new companies.
The MDI Biological Laboratory has a long and vigorous record of educating Maine students and professionals — and those from around the world — in the life sciences. In recent years, these offerings have increasingly focused on innovation- and entrepreneurship-based learning in recognition of the fact that science has to go hand-in-hand with training in these areas if discovery is to be translated into enterprises that improve health and create 21st century jobs.
Unique Research Approach
Referred to as the “vanguard of 21st century healthcare” by the U.S. Department of Health and Human Services, regenerative medicine is revolutionizing how we think about and treat disease. While the vast majority of research in this field is focused on developing stem cell, gene and tissue engineering therapies, these approaches have not yet delivered on their promise despite nearly two decades of research. The MDI Biological Laboratory approaches regenerative medicine differently by asking, “What if restoring the function of damaged tissue could be accomplished simply by taking a drug?”
MDI Biological Laboratory scientists are focused on the discovery and development of small molecules that activate the body’s innate ability to repair damaged tissue. This approach is founded on their extensive expertise in regenerative biology, the study of diverse regenerative animal models, genomics, big data analytics, integrative physiology, comparative and evolutionary biology, computational modeling and drug development. They are defining the gene regulatory networks and signaling pathways underlying heart, nerve and limb regeneration, wound healing, immune responses to injury, stem cell function and the loss of regenerative capacity with age.
It takes audacity to pursue unconventional approaches in regenerative medicine. But scientists at the MDI Biological Laboratory believe that solving the mysteries of regeneration is the key to preventing and curing countless diseases, to restoring function after traumatic injury and to slowing or reversing the degenerative changes that occur with age.
This approach — asking big questions, bringing new perspectives to problem-solving and using unconventional research models — works. In just three years and with minimal financial investment, MDI Biological Laboratory scientists have identified three drug candidates. The lead candidate, MSI-1436, stimulates heart muscle regeneration, improves heart function and increases survival following heart attack in animal models. The institution is seeking to move these drugs into the clinic through a spin-off, Novo Biosciences. MSI-1436 has already been found to be well tolerated by humans in Phase 1a and 1b clinical testing for an unrelated application.
The drug candidate has a huge potential market: cardiovascular disease is the world’s leading killer, taking the lives of 17.5 million people annually worldwide and disabling millions more.
Scientists at the MDI Biological Laboratory have also identified two drug candidates for the treatment of chemotherapy-induced peripheral neuropathy, a painful, disabling condition that affects at least 20 million Americans. In severe cases, patients may have to terminate chemotherapy prematurely, depriving them of the full benefits of treatment and possibly jeopardizing their lives. The MDI Biological Laboratory is also seeking to move these drugs into the clinic.
Historical Roots
The MDI Biological Laboratory’s 21st century focus has its roots in the 19th century. Much of the institution’s history is tied to a deep appreciation of the diversity of the natural world and the recognition that nature has already developed solutions to some of biology’s most pressing problems. The MDI Biological Laboratory (originally known as the Harpswell Laboratory) was established as a summer research station in 1898 in South Harpswell, Maine, in the midst of a new era of scientific thinking. The publication of Darwin’s Origin of Species about 40 years earlier had stimulated enormous interest in the natural world, leading to the formation of a new branch of science known as evolutionary biology.
The move to Bar Harbor was inspired by a desire to explore and protect the beautiful Maine coast. In the early 20th century, philanthropist George B. Dorr joined Harvard University President Charles W. Elliot and Standard Oil heir John D. Rockefeller Jr. and others in a mission to preserve Mount Desert Island — 140 miles to the north of the South Harpswell laboratory. As part of his plan for what would become Acadia National Park, Dorr envisioned a laboratory dedicated to the study of nature. In 1921, he convinced the South Harpswell laboratory to relocate to Bar Harbor. The name was changed two years later to the Mount Desert Island Biological Laboratory.
Modern Focus
The initial focus on exploring nature’s diversity shifted in the 20th century to understanding the physiology of the organisms themselves, especially as it related to human health. During this period, much important research was conducted at the MDI Biological Laboratory, including discoveries about kidney function that led to the development of kidney dialysis, discoveries about cell transport that improved drug delivery, research that contributed to the development of a glaucoma drug and research on the health impacts of environmental toxins like DDT and crude oil.
In the 21st century, the development of sophisticated genomics tools such as high throughput genetic screening enabled a shift from the study of the whole organism to the study of the molecular pathways underlying disease processes. With these advances, the MDI Biological Laboratory transitioned from a summer research station to a vibrant year-round research facility and, drawing heavily upon its comparative biology approach to discovery, shifted its focus to the translation of basic discoveries into regenerative medicine therapies to improve human health.
The institution also solidified its commitment to research training, establishing the Maine INBRE program (IDeA Network of Biomedical Research Excellence), a National Institutes of Health (NIH)-funded program that prepares Maine students for careers in biomedical research. Since its inception in 2001, the program has provided training for more than 2,100 students. With the launch of the Center for Science Entrepreneurship, the MDI Biological Laboratory will significantly expand the number of students trained each year and build upon its legacy of helping to develop a thriving science- and technology-based economy in the region.
In addition to Maine INBRE, the MDI Biological Laboratory offers numerous courses and conferences that attract students, scientists, science professionals and physicians from all over the world. Known for its signature courses on the use of comparative biology approaches to understanding aging and regenerative biology and, most recently, for its course on organoid technology, the institution continues to draw on its unique history to offer contemporary solutions that allow scientists to model diseases and screen for drugs that one day could lead to the repair of defective tissue.
Research Highlights
The MDI Biological Laboratory supports 10 research faculty and 26 research support staff. The goal is 15—18 independent research laboratories with a total staff of 120
within the next decade. Below are brief descriptions of faculty members who are conducting research in regenerative medicine and their work. Five faculty members are also featured in related interviews.

James A. Coffman, PhD, Associate Professor
James A. Coffman, PhD, is director of Maine INBRE, a NIH-funded program to promote biomedical training and research. He also studies the mechanisms underlying a predisposition to inflammatory diseases and accelerated aging caused by chronic early life stress. His research examines how such exposure programs the development of the immune system and the long-term effects on tissue repair and regeneration. He studies zebrafish because the cortisol-mediated stress response is conserved between zebrafish and humans and because zebrafish are amenable to detailed mechanistic studies of early development and adult regeneration.
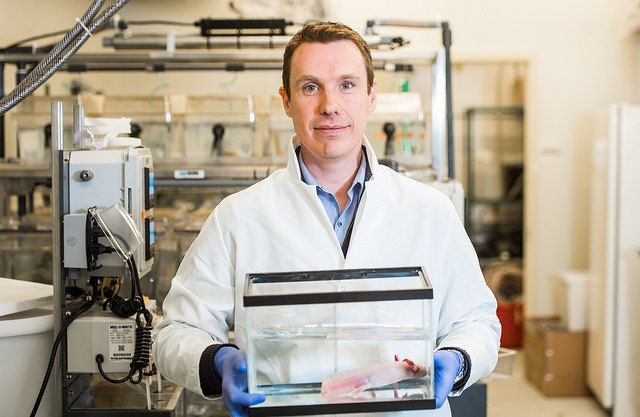
James Godwin, PhD, Research Scientist
James Godwin, PhD, studies the role of the neurological and immune systems in heart and limb regeneration in the axolotl, a salamander. He was formerly at the Australian Regenerative Medicine Institute, which partners with the MDI Biological Laboratory to promote regenerative medicine research and education. The two institutions are among the few in the world that are studying highly regenerative organisms in order to gain insight into regeneration in humans. Godwin holds a dual appointment with The Jackson Laboratory, also in Bar Harbor, where he studies mice, which, like humans, have a limited capacity for regeneration. The comparison offers insight into how to reverse-engineer regenerative processes in humans.

Hermann Haller, MD Professor, Director of the Department of Nephrology and Hypertension, Hannover Medical School, Hanover, Germany
Professor Herman Haller, MD, is an internationally acclaimed expert in the areas of kidney disease, hypertension and renal transplantation. In research at the MDI Biological Laboratory and in Germany, he is studying the molecular mechanisms of kidney regeneration in animal species such as the skate and the zebrafish that maintain the ability to regenerate organs. He is using this knowledge in a quest to create new kidneys in humans. As director of an internationally renowned clinic for renal disease and hypertension, he has also made important contributions in the fields of diabetic nephropathy, pregnancy-associated disease, renal vascular hypertension and transplantation.
Read the RegMedNet interview with Professor Herman Haller

Vicki P. Losick, PhD, Assistant Professor
Vicki P. Losick, PhD, has identified a healing mechanism in fruit flies and mice that she has called Wound-Induced Polyploidy (WIP). Wound healing has traditionally been thought to rely on cell division to regenerate cells lost to injury, aging or disease. But Losick has found that organisms can also replace cells by enlarging existing cells. She is studying why these extra-large cells, called polyploid cells, arise and how they function during stress responses. The goal of her research on the molecular mechanisms underlying WIP is to improve wound healing in humans through the development of therapies to stimulate the generation of these extra-large cells. In particular, she is studying the role of WIP in cornea endothelial injury.
Read the RegMedNet interview with Vicki P. Losick

Sandra Rieger, PhD, Assistant Professor
Sandra Rieger, PhD, is studying sensory nerve regeneration in peripheral neuropathy, a potentially disabling condition that causes numbness, tingling, temperature sensitivity and pain in the extremities. Peripheral neuropathy can be caused by chemotherapy, diabetes, alcohol, infectious disease and other conditions. Rieger uses zebrafish to study the molecular mechanisms underlying peripheral neuropathy induced by paclitaxel, a chemotherapeutic agent. 60—70% of patients treated with paclitaxel develop peripheral neuropathy. She has identified two drug candidates that can prevent and repair nerve damage in zebrafish by inhibiting an enzyme in the epidermis. Her goal is to move these drugs into the clinic.
Read the RegMedNet interview with Sandra Rieger

Aric Rogers, PhD
Aric Rogers, PhD, studies how gene expression is remodeled under conditions that extend lifespan in the roundworm, C. elegans. In particular, he studies the genetic mechanisms underlying the life-prolonging effects of dietary restriction — or a dramatic reduction of calories — which is known to extend lifespan in a range of species. He also studies the connection between a delay in the onset of age-related diseases — including diabetes, cancer and neurodegeneration — and lifespan-extending genetic variations and environmental conditions. The goal of his research is to understand how life-extending interventions work across different species and to apply what is learned to the extension of healthy human lifespan.
Read the RegMedNet interview with Aric Rogers

Kevin Strange, PhD, President and Professor
Kevin Strange, PhD, studies proteostasis network biology, or the cellular machinery that maintains cellular quality control. Using the roundworm, C. elegans, he studies how cells detect, degrade and repair damaged protein. His research has demonstrated that protecting the structure and function of proteins is an essential part of how cells survive stressful environments. His findings are important for understanding how cells cope with a range of stressors, including aging. Strange is also the CEO and co-founder of Novo Biosciences and the co-inventor of MSI-1436, a drug candidate for the treatment of damaged heart muscle tissue and other conditions.

Dustin Updike, PhD, Assistant Professor
Dustin Updike, PhD, uses the roundworm, C. elegans, to study germ cells, which are the progenitors of reproductive cells and the only cells in sexually reproducing animals that give rise to all of the cell types of each subsequent generation, a phenomenon called totipotency. He studies how germ granules, organelles found just outside the nucleus of germ cells, help maintain totipotent and immortal properties and how these mechanisms can be applied to unlocking stem cell-like properties in other types of cells. Since cancer cells share the ability to self-renew, his research also has implications for the prevention and treatment of cancer.

Voot P. Yin, Assistant Professor
Voot P. Yin, PhD, studies tissue and organ regeneration in zebrafish. He is seeking to understand how zebrafish regenerate damaged tissue so therapies can be developed to reawaken the dormant genetic codes for regeneration in humans. Yin has discovered that microRNAs play a central role in the initiation and propagation of this process. He is also the co-inventor of MSI-1436, a drug candidate that has been shown to enhance the regeneration of complex heart muscle tissues. His groundbreaking work led to the co-founding with MDI Biological Laboratory President Kevin Strange, Ph.D., of Novo Biosciences for the purpose of commercializing MSI-1436 and other regenerative medicine therapies. His research has implications for heart muscle regeneration, limb regeneration, muscular dystrophy, prosthetics and wound healing.