Manufacturing exosomes at a commercial scale: an interview with Jorge L Escobar Ivirico

 Jorge Escobar is a senior research scientist in the applications lab of Eppendorf (CT, USA). He joined the company in 2019 and has worked on applied research focused on efficient expansion of adherent and non-adherent cells (e.g., stem cells, adult primary cells) in cell culture bioreactors.
Jorge Escobar is a senior research scientist in the applications lab of Eppendorf (CT, USA). He joined the company in 2019 and has worked on applied research focused on efficient expansion of adherent and non-adherent cells (e.g., stem cells, adult primary cells) in cell culture bioreactors.
After his PhD in Biomedical Engineering at the Polytechnic University of Valencia (UPV; Spain), he gained experience in the fields of cell therapy and tissue engineering working as a researcher at the Center for Biomaterials and Tissue Engineering (UPV), Institute of Biomaterial Sciences – Helmholtz-Zentrum Geesthacht (Berlin, Germany) and Institute for Regenerative Engineering (University of Connecticut Health, CT, USA). Additionally, in 2017, he was promoted to Assistant Research Professor (Department of Chemical and Biomolecular Engineering, University of Connecticut). During his more than 10 years’ experience he is the author of more than 50 scientific publications including research papers, books chapters and applications notes, recipient of several awards and member of the Regenerative Engineering Society, European Biomaterials Society and Latin American Society of Biomaterials.
What benefits does acellular regeneration medicine, for example using exosomes, have over cell-based medicine?
The need for effective treatments for various unmet conditions prompted the regenerative medicine field to emerge more than 20 years ago as an alternative field that aims to restore specific tissues and organs in the human body of patients suffering from severe injuries or chronic conditions. Basically, adult stem cells from the same patient (autologous strategy) or from different patients (allogeneic strategy) were the vehicles used to restore damaged tissue by administering them to the site of injury. When implanting, it was thought that stem cells should migrate and integrate into the injury site and replace the patient’s own damaged tissue. However, limitations associated with cellular localization or the number of cells reaching the target tissue and cell viability work against the beneficial effects of tissue regeneration. Furthermore, from a safety perspective, the behavior of stem cells after transplantation into the human body is unpredictable.
New evidence indicates that the therapeutic benefits of stem cells are attributed to paracrine factors released by small extracellular vesicles (including exosomes) that are secreted by stem cells, enhancing their cell–cell communication. Following in the footsteps of other cell therapy strategies, stem cell-free therapy using mesenchymal stem cell (MSC)-derived exosomes can improve inter-cell signaling and reduce inflammatory processes that lead to cell regeneration, as well as minimize the risk of tumor formation and provide lower immunogenicity.
What are some of the most common mistakes you see researchers making in the production of exosomes?
This is an emerging field and no gold standards have been developed so far, thus new procedures and methodologies seem to appear almost daily. In addition to researchers making mistakes during exosome production, I see that scientists face great challenges, for example, in developing efficient formulations of the cell culture media necessary to improve cell growth and production of exosomes, new isolation procedures that offer advantages in terms of performance and cost–effectiveness and more accurate purification methods.
How does manufacturing exosomes differ to manufacturing cells? How are their needs different?
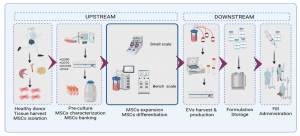
Figure 1. Example of an allogeneic stem cell-free therapy workflow. Created with biorender.com.
As a product of cells, the manufacture of stem cell-derived exosomes (during the upstream process) is dependent upon cell growth without altering certain cell behaviors and characteristics. Although exosome biogenesis is not well understood, recent studies suggest that changes in the cell phenotype (due to possible changes of the production parameters such as mass transfer, shear forces or pH during the cell growth) can alter the exosome yield, composition and function.
It is during the downstream process that the greatest differences between the production of cells and exosomes are observed. Most of the cell platforms used in exosome production are anchorage-dependent, so harvesting the cells requires a detaching process from a substrate. On the other hand, exosomes are secreted by the cell into the medium, so the collection of the cell-conditioned medium will be sufficient to initiate the exosome isolation and purification steps. Knowing the specifics of the target exosome subpopulation is very important in selecting the appropriate downstream process development.
How can stirred-tank bioreactors be incorporated into a commercial-scale exosome production process?
As you know, there is an increased interest in exosome research and large-scale production due to its therapeutic potential as a drug-delivery system and in disease diagnosis. Eppendorf is pioneering the implementation of single-use bioreactors to control and monitor exosome production and looks for a scalable process to increase exosome production throughput. Stirred-tank bioreactors offer the possibility to precisely monitor and control all relevant process parameters. Therefore, the effects of individual parameter adjustments can be directly seen, analyzed and used to further improve the process. Recently published results from the group of Robert Zweigerdt can be shown as an example here. Using step-by-step analysis of the effects of different parameters, they increased the yield of human pluripotent stem cells in a 250 ml single-use bioreactor to 35 × 106 cells/ml.
What exosome characteristics should be monitored to ensure a high-quality, stable finished product?
As mentioned previously, cell growth in stirred-tank bioreactors should be monitored to avoid changes in the desired exosome composition. Exosomes present a complex architecture in terms of proteins, lipids, nucleic acids and other components that depend on the cell of origin. Among proteins, tetraspanins (CD63, CD9, CD81 and CD82) play a key role in cell penetration invasion and fusion events. Most of these proteins have also been used as references in the development of ultracentrifugation-free isolation and quantification methods. In addition, it is important to highlight that according to the International Council for the Harmonization of Technical Requirements for Pharmaceutical Products for Human Use (ICH), several considerations should be addressed during the establishment of standard operating procedures regarding manufacturing, characterization, storage and distribution of exosome-based therapies, among which stand out: 1) evaluation of large-scale manufacturing, 2) the route of exosome application, 3) risk assessment regarding exosome-based therapeutic strategy (autologous or allogeneic), among others.
How do you think exosome use might evolve in the future, and how will this affect the needs of therapy developers?
MSC-derived exosomes have gained enormous interest recently due to their ability to mediate as intercellular messengers. In fact, their unique characteristic makes them attractive for different biomedical applications such as cancer diagnosis, gene therapy, drug delivery, vaccine development and cell therapy, and it is in these research areas where, from my point of view, the main contributions will be made in the near future. Furthermore, therapy developers will need to tailor their stem cell-free therapy workflow in accordance with new exosome production requirements, primarily in developing effective ways to perform exosome harvesting and, at a certain point, setting gold standards for the purification and characterization of exosomes.
How do vendors such as Eppendorf ensure they are addressing the needs of researchers in emerging fields such as acellular regenerative medicine?
For many years, our company has provided key resources to address stem cell workflow challenges for clinical research and development. Our portfolio of products and services is designed to support and accelerate the journey from discovery to the clinic, and the same strategy is being used in stem cell-free therapy or acellular regenerative medicine products. This is the case with our bioreactor systems that offer precisely controlled environments for stem cell culture and exosome production. Eppendorf is a pioneer and reference in the field through generating knowledge and applications for our customers by using our technology in the production of exosomes. We work closely together with our customers to understand their needs. One example for this collaboration is the development of our eight-blade impeller, which was developed especially for stem cell cultures. This impeller keeps cells in solution already at low stirring speeds, ensuring stable process conditions while maximizing the yield.
Disclaimer
The opinions expressed in this interview are those of the interviewee and do not necessarily reflect the views of RegMedNet or Future Science Group.
This article is part of the RegMedNet Spotlight on acellular regenerative medicine. Click here to view the full feature and discover expert insights on this >>>
In association with: