Peptide-based supramolecular gels for tissue engineering
Peptide nanotechnology and supramolecular chemistry now offer a toolbox for the design of peptide-based biomaterials with tunable properties useful for regenerative medicine.
By Ayala Lampel1 and Rein V Ulijn1,2
1Advanced Science Research Center (ASRC), City University of New York, 85 St Nicholas Terrace, New York, NY 10031, USA
2Hunter College, Department of Chemistry, City University of New York, 695 Park Avenue, New York, NY 10065, USA
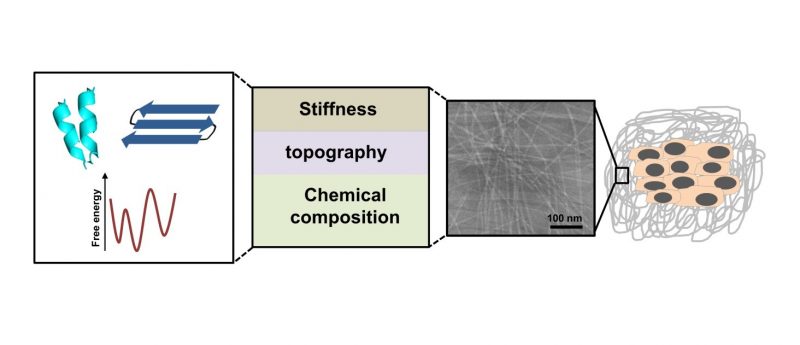
Over the past two decades, peptide-based supramolecular gels have become widely used for biomedical applications, including repair and regeneration of damaged tissues, cell support scaffolds and localized drug delivery. Peptides offer an attractive platform for biomaterials fabrication since they are easy to synthesize, present a diverse set of chemical groups and from clinical perspective — are biocompatible and biodegradable [1]. Peptide building blocks can be designed to self-assemble through various non-covalent interactions such as hydrogen bonding, Ï€—Ï€ interactions, hydrophobic and electrostatic interactions into a network of well-ordered nanostructures that forms the supramolecular hydrogel. These nanostructures, typically one-dimensional fibers, share similar structural aspects with the natural extracellular matrix and can be designed to mimic its bioactivity by incorporating specific functional signals.
Here, we will highlight some of the main principles for the design of supramolecular peptide hydrogels for regenerative medicine, starting with the main properties that affect cell fate and behavior, different approaches for building block design, and ending with future direction for biomaterials design.
Key properties of supramolecular biomaterials
The natural extracellular matrix provides a physical support for cells and tissues, and regulates a variety of bioactive signals that affect cell fate and behavior [2—3]. Peptide-based supramolecular matrices are useful alternative to the natural matrices since they can mimic these essential structural and functional properties but are completely defined and much simpler in composition. Specifically, the stiffness of artificial matrices was shown to influence the proliferation, differentiation, and migration of cells [2]. The mechanical properties of supramolecular gels depend on the properties of the individual fibers and the level of crosslinking between the fibers. Thus, the matrix stiffness can be tuned according to the design of the peptide building blocks, their concentration, or by changing solution conditions such as pH, presence of multivalent cations, and ionic strength. Moreover, unlike chemically crosslinked polymers, the supramolecular crosslinking between fibers is non-covalent and reversible and therefore the stiffness of peptide-based matrices is a dynamic property. In a similar manner, the topography of the supramolecular matrix has also considerable impact on cell behavior [2—3]. This includes the shape and surface texture of the nanostructures, their packing geometry and the architecture of the nanostructures network. These properties can also be tuned based on the molecular design.
One of the key advantages of peptide-based supramolecular gels for tissue engineering is their ability to present bioactive signals, which can be achieved by incorporating the bioactive component into the peptide sequence or by co-assembling the peptide with the bioactive signal. A smart molecular design should result in a display of bioactive signals that are accessible to cells, while keeping the integrity of the self-assembled nanostructures. Using this strategy, peptide amphiphile-based scaffold presenting the laminin-derived epitope IKVAV was shown to selectively induce differentiation of neuronal stem cells into neurons, and the fibronectin-derived sequence RGDS was incorporated into peptide building block sequences of different supramolecular gel systems to promote cell adhesion [4].
Peptide design approaches
The most common self-assembly motif used for the design of peptide-based gels is β-sheet hydrogen bonding, where the β-sheet forming peptides typically assemble into fibrillar nanostructures [1]. Incorporating aromatic or hydrophobic amino acids into the peptide sequence results in additional assembling inducing motifs (Ï€—Ï€ or hydrophobic interactions) that drive the self-assembly and enables the use of simpler, shorter peptides. These include peptides conjugated with non-peptidic moieties such as peptide amphiphiles [4], and short peptides gelators [5]. Recent study by our group showed that the position of specific amino acids (aromatic, charged) within short peptides is critical for their assembly propensity [5].
Additional approaches for the design of hydrogel-forming peptides are based on the α-helical coiled- coil motif and electrostatic interactions. The first approach relies on α-helical peptides with sticky ends that promote longitudinal assembly and bundling of the coiled-coil fibers [6], whereas in the second approach the peptide is designed to self-assemble by the ionic interactions between alternating sequences of positively and negatively charged amino acids [7].
Future perspective
A recent study by the Stupp group shows that a function of peptide supramolecular system is dictated by positions in its energy landscape [8]. Given the same molecular design, altering the assembly conditions (ionic strength and temperature) shifted the balance between two competing forces, β- sheet forming hydrogen bonding and electrostatic repulsion which changed the energetic “well” of the assemblies. Moreover, assemblies at different thermodynamic minima had completely different functionality, the thermodynamically favored product was found to be bioactive whereas the unfavorable product was cytotoxic. This study suggests a new opportunity for the design of functional supramolecular biomaterials based on environmental and energetic considerations rather than merely on molecular design.
References
- Webber MJ, Appel EA, Meijer EW, Langer R. Supramolecular biomaterials. Nat. Mater. 15(1), 13—26 (2016).
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751), 1139—1143 (2005).
- Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nanotopography and integrin—matrix interactions to influence stem cell fate. Nat. Mater. 13(6), 558—569 (2014).
- Pérez CMR, Stephanopoulos N, Sur S, Lee SS, Newcomb C, Stupp SI. The powerful functions of peptide-based bioactive matrices for regenerative medicine. Ann. Biomed. Eng. 43(3), 501—514 (2015).
- Frederix PW, Scott GG, Abul-Haija YM et al. Exploring the sequence space for (tri-) peptide self-assembly to design and discover new hydrogels. Nat. Chem. 7(1), 30—37 (2015).
- Banwell EF, Abelardo ES, Adams DJ et al. Rational design and application of responsive α-helical peptide hydrogels. Nat. Mater. 8(7), 596—600 (2009).
- Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self- complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. USA 90(8), 3334—3338 (1993).
- Tantakitti F, Boekhoven J, Wang X et al. Energy landscapes and functions of supramolecular systems. Nat. Mater. doi:10.1038/nmat4538 (2016) (Epub ahead of print).