Retinitis pigmentosa cell therapy receives Regenerative Medicine Advanced Therapy designation
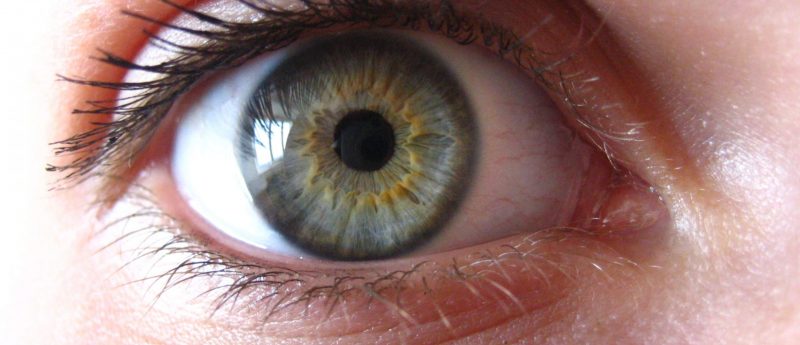
jCell, from jCyte, is an investigational therapy that contains human retinal progenitor cells to rescue damaged retinal cells.
An investigational cell therapy to treat retinitis pigmentosa (RP) has received Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA. Drugs designated as RMATs are recognized to address an unmet need in serious or life-threatening conditions and are given additional support by the FDA in order to accelerate their development, review and approval.
“This is uplifting news for patients with RP,” said jCyte (CA, USA) co-founder, Henry Klassen. “At this point, there are no therapies that can help them avoid blindness. We look forward to working with the FDA to speed up the clinical development of jCell.”
RP is a degenerative ophthalmic disease that affects retinal rods and cones; it usually strikes sufferers before the age of 20, causing complete blindness by the time they reach 40. jCell, from jCyte, contains human retinal progenitor cells which release neurotrophic factors that could encourage regeneration of damaged retinal tissue. It will be delivered via an intravitreal injection.
“This is among the first ophthalmic products to receive RMAT designation,” said jCyte CEO, Paul Bresge. “We are gratified by the FDA’s interest in the therapeutic potential of jCell and greatly appreciate their decision to provide extra support. We are now conducting the all-important trials to validate that confidence.”