The technological developments that have allowed organoids to become a viable research tool and the remaining challenges in their production
In this exclusive editorial, Hugo de Jonge, Erasmus University Medical Center, reviews the state of the art in organoid production and continuing obstacles in their manufacture.
Hugo R. de Jonge1
1Department of Gastroenterology & Hepatology, Erasmus University Medical Center, Rotterdam, The Netherlands
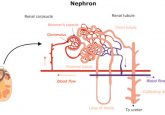
Stem cell or tissue-derived multi-cellular 3D organoids, also referred to as mini-organs or organs in a dish, provide superior in vitro models for studying human diseases and performing drug screening, as compared to conventional 2D cell culture systems. These complex, self-organizing structures can grow from embryonic stem cells, induced pluripotent stem cells (iPSCs) or organ-specific adult stem cells (ASCs), and have been established for multiple organs including intestine, liver, pancreas, stomach, kidney, lung, salivary gland and brain (1-3). Patient-derived organoids offer great promise for application in the field of translational medicine, in particular for autologous transplantation and tissue regeneration purposes (4-6). They have also been used advantageously for personalized mutation and drug screening in cancer and hereditary diseases such as cystic fibrosis, and for studying infection or inflammatory diseases involving host-pathogen interactions (2,7-12).
The organoid technology field has advanced enormously as a result of two key discoveries. One is the molecular identification of cell growth and differentiation factors, and their receptors that mimic embryonic developmental cues, and allow PSCs to differentiate into almost any mammalian cell type. The other one, the finding by the Clevers group in Utrecht that adult Lgr5+ stem cells from the intestine, grown 3D in the presence of basement membrane matrix and a defined set of niche factors, can develop into ever-expanding, genome-stable 3D organoids that resemble the structure and function of the original intestinal epithelium (13-16). As anticipated from the identification of Lgr5+ as a marker of ASCs in multiple other tissues, a similar approach proved successful in generating organoids or tumoroids from virtually any epithelial organ or tissue (2,3).
Gene-editing techniques such as CRISPR/Cas9 could potentially be applied to organoids derived from patients with monogenic defects, such as cystic fibrosis, polycystic kidney disease, microvillus inclusion disease and alpha1-antitrypsin deficiency to generate healthy tissue for transplantation (17). In vivo functional engraftment has already been demonstrated for intestinal, liver, salivary, kidney and beta-cell enriched pancreatic organoids in mouse models (2,18).
Due to the high efficiency of establishing organoid models from different tissues and diseases, such as cancer, organoid technology allows the generation of large living biobanks of tumor organoids that are amenable for middle-throughput drug screens and may allow personalized therapy design, as a complement to cell line and xenograft-based drug studies (7,19).
However, despite its marked promise for disease modeling, development of novel therapies, and regenerative medicine, stem cell derived organoid technology faces many remaining challenges. First, most currently generated organoids mainly contain epithelial cells and are void of the many different cell types that are essential for the morphology and functionality of the whole organ. Organ-on-a-chip technology may in part overcome this limitation, as exemplified by the “breathing” lung-on-a-chip that recapitulates the alveolar-capillary interface by co-culturing human alveolar epithelial cells and capillary endothelial cells on opposite sides of a flexible, porous, ECM-coated membrane.
Culture medium containing immune cells is passed through the vascular channel and air through the upper channel to create an air-liquid interface (20). Biomechanical stretch and strain can be mimicked in this device by rhythmic contractions of the flexible membrane. Protocols to grow organoids as polarized monolayers of epithelial cells on membranes or filters are already in place for the intestine, gall bladder, liver and pancreatic ducts (21). These 2D cultures also abolish the need for intraluminal microinjection of single organoids and facilitate studies of host-microbe or virus interactions or the action of microbial enterotoxins because the luminal surface is fully exposed to the mucosal medium (22,23).
Secondly, before considering the use of iPSC-derived organoids for transplantation/regenerative medicine in human patients, the current protocols for expansion, reprogramming and differentiation of iPSCs in long-term cultures need further improvement to minimize the risk of oncogenic cellular mutations and teratoma, or tumor formation, in the patient. Whole-genome sequencing data and epigenetic studies are needed to address this concern.
Thirdly, the current, almost universal dependency of organoid culture on the use of Matrigel as a replacement for the function of the extracellular basement matrix in providing structural support and survival signals to the epithelial cells hampers clinical application, considering its origin from a mouse sarcoma cell line, its poorly defined composition and its mechanical rigidity after plating. Next-generation “designer matrices” such as hybrid polyethylene glycol hydrogels or microengineered collagen scaffolds, combined with a well-defined set of laminins, may better fulfill the niche requirements of organoids and may be customized for a specific type of tissue/organoid (2,6).
Finally, the future success of autologous transplantation of genetically repaired or in vitro expanded organoids in patients, abolishing the need for allogeneic donors and for lifelong immunosuppression, is dependent on a significant improvement in engraftment efficiency. Perhaps one approach to reach this goal is the combined use of organoid engraftment techniques and pharmacotherapy with small molecules that are capable of stimulating tissue regeneration, such as the ones recently discovered at the MDI Biological Laboratory (22).
This and many other aspects of present and future applications of organoids in the regenerative medicine field will be the focus of the new Applications of Organoid Technology course scheduled for May 27—June 2 at the MDI Biological Laboratory in Bar Harbor (ME,USA). For more on the course, please email [email protected] or visit the website at mdibl.org/education/courses/.
Note: Dr. Sylvia Boj, Foundation Hubrecht Organoid Technology, Utrecht, The Netherlands, is acknowledged for critical reading of the manuscript.
References
- Lancaster MA, Knoblich JA. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science, 345:1247125 (2014)
- Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 23: 393-410 (2017)
- Gehart H, Clevers H. Repairing organs: lessons from the intestine and liver. Trends in Genet. 31: 344-351 (2015)
- Huch M, Boj SF, Clevers H. Lgr5+ liver stem cells, hepatic organoids and regenerative medicine. Regen. Med. 8: 385-387 (2013)
- Dye Dedhia P, Miller AJ, Nagy, MS, White ES, Shea LD, Spence JR. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. eLife. 5: e19732 (2016)
- Nakamura T, Sato T. Advancing intestinal organoid technology toward regenerative medicine. Cell Mol. Gastroenterol. Hepatol. 5: 51-59 (2017)
- Van de Wetering M, Francies HE, Francis JM et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 161: 933-945 (2015)
- Dekkers JF, Wiegerinck CL, de Jonge HR et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 19: 939-945 (2013)
- Ikpa PT, Bijvelds MC, de Jonge HR. Cystic fibrosis: toward personalized therapies. Intl J Biochem. Cell Biol. 52:192-200 (2014)
- Dekkers JF, Berkers G, Kruisselbrink E et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 8(344):344ra84. doi: 10.1126 (2016)
- Finkbeiner SR, Zeng X-L, Utama B, Atmar RL, Shroyer NF, Estes MK. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio. 3(4): e00159-12 (2012)
- Hill DR, Spence JR. Gastrointestinal organoids: understanding the molecular basis of the host-microbe interface. Cell Mol. Gastrenterol. Hepatol. 3: 138-149 (2017)
- Barker N, van Es JH, Kuipers J et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449: 1003-1007 (2007)
- Sato T, Vries RG, Snippert HJ et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459: 262-265 (2009)
- Sato T, Stange DE, Ferrante M et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 141: 1762-1772 (2011)
- Blokzijl F, de Ligt J, Jager M et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature. 538(7624):260-264 (2016)
- Schwank G, Koo B-K, Sasselli V et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 13: 653-658 (2013)
- Yui S et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 18: 618-623 (2012)
- Vlachogiannis G, Hedayat S, Vatsiou A et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 359(6378):920-926 (2018)
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 328: 1662-1668 (2010)
- Zachos NC, Kovbasnjuk O, Foulke-Abel J et al. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J Biol. Chem. 291:3759-3766 (2016)
- Van Dussen KL, Marinshaw JM, Shaikh N et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 0:1-10.doi:10.1136 (2014)
- Kim HJ, Li H, Collins JJ, Ingber DE. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. PNAS. 113: E7-E15 (2016)
- Noel G, Baetz NW, Staab JF et al. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Nat. Sci. Rep. 7: 45270 (2017)
- Strange K. Looking beyond stem cells and tissue engineering: What if lost and damaged tissues and organs could be regenerated simply by taking a drug? Regenerative Medicine Network: Spotlight on MDI Biological Laboratory, Research (March 2017)