Regenerative DNA-based medical revascularization to treat refractory angina: an interview with Christopher Reinhard

In this interview, Christopher Reinhard, Angionetics, explains the challenges of commercializing gene therapies and the potential for regenerative cardiac therapeutics.
In this interview, Christopher Reinhard, Angionetics (CA, USA), explains why refractory angina was chosen as a target for his novel regenerative gene therapy. He also offers his thoughts on the challenges of commercializing gene therapies.

Christopher Reinhard
Christopher Reinhard is the co-founder, President and Chief Executive Officer of Angionetics. Over the past 15+ years, he has focused on the clinical and commercial development of innovative gene-based cardiovascular therapeutics and medical devices. During this period, he also concurrently held C-level executive positions at Schering (cardiovascular gene therapy operating unit), Cardium and Collateral Therapeutics, Activation Therapeutics (DE, USA), InnerCool Therapeutics, To Go Brands, MedPodium and Artes Medical (all CA, USA), and prior to that held executive positions at Fisher Scientific Group and the Henley Group.
Can you introduce yourself and briefly describe your career to date and introduce Angionetics?
I am Christopher Reinhard, the co-founder, President and Chief Executive Officer of Angionetics (CA, USA). For over 15 years, I have principally focused on the clinical and commercial development of innovative therapeutics and medical devices. During this time, I have been responsible for continued advancement of Angionetics’ Generx [Ad5FGF-4] cardiac angiogenic gene therapy from a scientific lab bench at the University of California-San Diego (CA, USA) into late-stage Phase 3 clinical study.
Since its formation, I am pleased to report that Angionetics has received FDA clearance for a new USA Phase III clinical study as a one-time treatment for patients with myocardial ischemia and refractory angina due to advanced coronary artery disease and we entered into our first international strategic partnership with Huapont Life Sciences to market and sell Generx in Mainland China. We are also pleased to report that the FDA recently granted the Generx program Fast Track designation.
What causes angina?
As described by the National Heart, Lung and Blood Institute (a division of the National Institutes of Health), angina—recurrent or severe chest pain or discomfort that profoundly limits physical activity and quality of life—is usually a symptom of advanced coronary heart disease. This is a disease in which a waxy substance called plaque builds up inside the coronary arteries. These arteries supply oxygen-rich blood to your heart muscle. This means that the underlying causes of angina generally are the same as the underlying causes of coronary heart disease. Many factors can trigger angina pain, depending on the type of angina you have. Physical exertion is the most common trigger of “stable” angina.
How is angina treated?
Upon an initial diagnosis of angina, patients generally are prescribed the time-honored anti-anginal oral drug therapy. These drugs may be taken episodically when an angina attack occurs, usually as a result of physical exertion, or taken daily to temporarily relax the heart’s major coronary arteries to increase blood supply or to reduce the heart’s need for oxygen-rich blood. Over time, however, in some patients, drug therapy becomes no longer effective as coronary artery disease progresses.
Based on current practice, with disease progression some patients may qualify for a “mechanical” revascularization procedure that could include a catheter-based, percutaneous coronary intervention and stent implantation, or a more invasive procedure called coronary artery bypass surgery. These procedures are designed to restore blood flow through the heart’s three major coronary arteries to increase oxygen supply to heart muscle.
In recent years, there has also been a growing awareness within the medical community that “early” mechanical revascularization, without an observable angiographic risk or an immediate risk of heart attack, may not convey any prophylactic mortality benefit. Because of this new understanding, together with the widespread use of statin therapy and more healthy lifestyles, over the past 10 years, stent procedures have fallen by almost 30% and coronary artery bypass surgery has dropped by almost 50%.
In addition, approximately 20—30% of patients who have had a mechanical revascularization procedure will continue to suffer from angina chest pain, which hampers their quality of life and restricts normal physical activity levels.
Based on this convergence, we now identify patients with “refractory angina” as patients who experience persistent angina and are:
- no longer responsive to optimal medical therapy and would not benefit from early prophylactic mechanical revascularization, or
- who continue to experience chronic angina following mechanical revascularization, without further treatment options.
In the United States, it is estimated that there are about 1.0 million patients with refractory angina. With the aging baby boom population, and increased lifespans, we expect that this patient population, with a significant unmet medical need, will continue to expand in the years ahead. Based on this convergence of outcome research and market demographics, we have focused our Generx clinical research activities on patients with refractory angina.
How does Generx address these patients?
In contrast to the two treatment approaches that I summarized above, Generx represents a third approach, which we call “medical revascularization.” Our Generx product candidate is designed for one-time catheter-based administration to stimulate and promote the heart’s innate natural ability to grow new blood vessels (through a process known as angiogenesis) or enlarge existing microvessels (through a process known as arteriogenesis), in regions with diminished blood flow due to coronary artery disease.
It has long been known that the human heart has the innate capacity to remodel in response to advancing coronary artery disease. As plaque builds up in the heart’s three major arteries as I described above, some genetically privileged patients with heart disease begin to grow small collateral blood vessels in an effort to overcome restricted blood flow and improve cardiac perfusion. While eventually the disease will overrun this angiogenic process, researchers have long wondered if this primal angiogenic healing response could be amplified and regulated through the design and development of angiogenic therapeutics. This is a key insight and the driving force behind our Generx angiogenic gene therapy development program.
How does Generx work?
The value of Generx as a therapy for angina involves its use of a specific regulatory gene, fibroblast growth factor-4 (FGF-4). FGF-4 is now known to activate vascular endothelial growth factors (VEGFs) and the cascade of events required to stimulate cardiac angiogenesis. The first thing we do is encase the 621 base pair human FGF-4 gene within a type of molecular delivery system known as an adenoviral vector. The resulting molecular package is then delivered into the heart as a one-time treatment during a standard angiographic procedure. During this procedure, which we call “cardiac microvascular gene therapy,” the therapy is injected into either the left or right coronary artery through a catheter that has been threaded up from the patient’s leg.
The biologic is injected in front of a balloon that briefly blocks blood flow, allowing the treatment to more easily leave the blood vessel and enter the cardiac muscle. Expression of FGF-4 protein promotes the development of new collateral vessels in the oxygen-starved sections of the heart and spurs existing collateral channels to grow into medium-sized arteries. The net result is increased blood flow and oxygen supply to the heart. Based on perfusion studies following mechanical revascularization and our internal studies of Generx medical revascularization, we have observed that angiogenic gene therapy may be able to increase perfusion at levels approximating 75% of that achieved following stent implantation and bypass surgery.
What are the challenges in developing gene therapies?
There are multiple challenges. One is determining which gene or genes are most likely to result in an effective therapy for a particular condition; for example, in the case of Generx, we focused on a gene that codes for a specific growth factor, which studies suggest is instrumental in promoting the development of new collateral vessels.
Another challenge involves determining how best to deliver the gene therapy into the body. Fortunately, we found that a type of virus, called adenovirus, works well as a delivery method for Generx. We also believe that a one-time treatment during a standard angiogram may serve as an effective treatment modality that fits well within the current practices of interventional cardiology and removes the need for repeat treatments. Of course, there are many more technical challenges associated with developing gene therapies, depending on the specific type of therapy being considered.
What are the obstacles for widespread use of gene therapies in clinical applications?
Some types of advanced cell therapies require that stem cells be removed from a patient and sent to a lab and modified, prior to being sent back to the hospital for infusion into the patient. This is an involved and costly process with many technical obstacles, such as ensuring that the cells remain viable during transport from hospital to lab and back again. Once more, we are fortunate that Generx exists as a therapy that can be applied during a standard angiogram-like procedure using an off-the-shelf product design.
Because of this process, the unique cost-effective manufacturing of gene-based therapeutics using bioreactors, and the potentially large-scale global market opportunity for cardiovascularcentric medical indications like refractory angina, we are seeking to be in a position to competitively price our Generx therapy in alignment with cardiac stents. At the present time, we believe that, on a global basis, especially outside the US, the high cost of cutting-edge therapeutics will become an obstacle to the widespread use of gene therapeutics, based on the accelerating trend of cost rationalization.
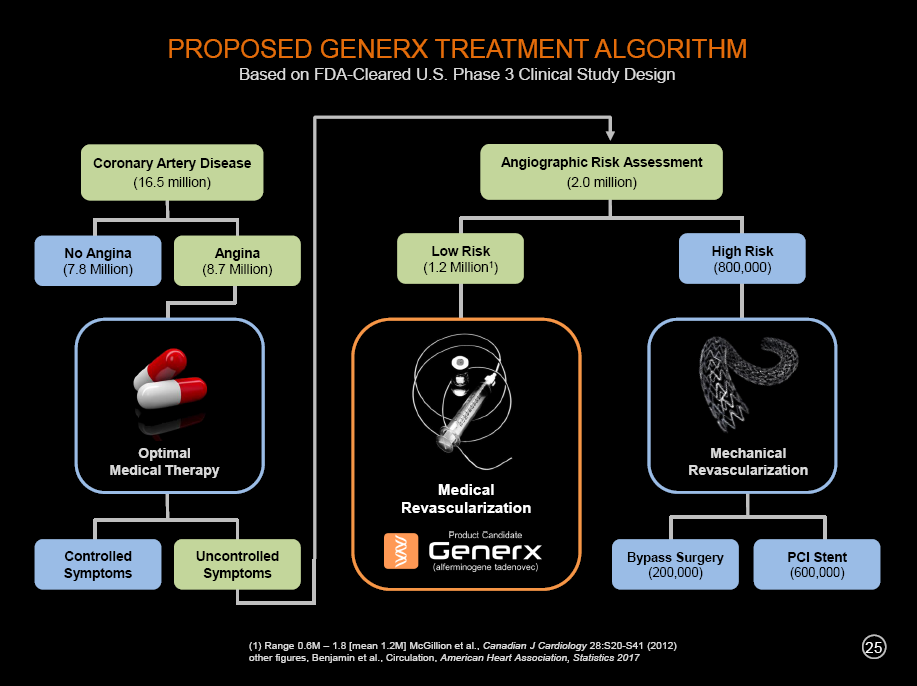
We are developing the Generx gene-based angiogenic therapy as a new and innovative therapeutic option for “medical revascularization” for use by the interventional cardiologist community in an important subset of patients with coronary artery disease. In the future, with clinical success, we would like to expand the algorithm for the treatment of angina and coronary artery disease to include (1) optimal drug therapy, (2) mechanical revascularization and (3) medical revascularization, a new treatment paradigm that would include our Generx angiogenic gene therapy product candidate. If successful, the chart above outlines what such a treatment algorithm might look like.
What does the future hold for Generx and Angionetics?
As I noted earlier, we are pleased that the FDA has recently cleared our Generx product candidate for a late-stage Phase III clinical study, called the AFFIRM study, in the United States as a new, single dose treatment for patients with myocardial ischemia and refractory angina due to advanced coronary artery disease. This study follows a series of positive earlier-stage studies involving this therapy.
The FDA also recently granted Fast Track designation for this study, which means it will take actions, as appropriate, to speed the development and review of a biologics license application (BLA) for product approval. Future studies are likely to elucidate the most promising therapies for cardiovascular angiogenic gene therapy and offer hope to many patients for whom angina is currently a source of deep concern causing significant negative impact on quality of life.
After almost two decades, we at Angionetics are looking forward to obtaining results from the AFFIRM study in the hope that it will get us closer to a viable and effective therapy for millions of patients with refractory angina and myocardial ischemia due to advanced coronary artery disease, a condition representing a significant unmet medical need for millions of patients worldwide. As we look to the future, we plan to focus on a next frontier and consider the exploration of our Ad5FGF-4 Generx product candidate for the potential treatment of millions of patients with cerebral ischemia and vascular dementia.
Acknowledgements & Disclosures
- Christopher Reinhard is Chief Executive Officer of Angionetics