Introducing CellGenix T-cell medium — early onset of T-cell expansion for faster T-cell therapy manufacturing
Achieve an early onset of T-cell expansion and sustained viability with the novel CellGenix (Freiburg im Breisgau, Germany) T-cell medium (CellGenix® TCM).
Serum-free T-cell therapy manufacturing
CellGenix (Freiburg im Breisgau, Germany) T-cell medium (CellGenix® TCM) offers a serum-free and xeno-free alternative for rapid expansion of functional human T-cells. Due to stable glutamine in the formulation it is ready-to-use for T-cell cultures without the need for supplementation with human serum or glutamine. Many current T-cell therapy protocols rely on the addition of human serum.
Eliminating the use of human serum will reduce the failure rate in your manufacturing process due to the high lot-to-lot inconsistencies of serum. Since human serum is a limited resource and might not be available in large quantities it is unsuitable for commercial scale manufacturing. Furthermore, the human origin of serum poses a certain risk of containing adventitious agents and therefore does not meet global regulatory guidelines.
Early onset of T-cell expansion and sustained viability
T-cell culture in CellGenix TCM results in high cell numbers early after activation and throughout cell culture with high viability. This early onset of T-cell expansion allows for faster T-cell therapy manufacturing, which can significantly reduce your cost of goods.
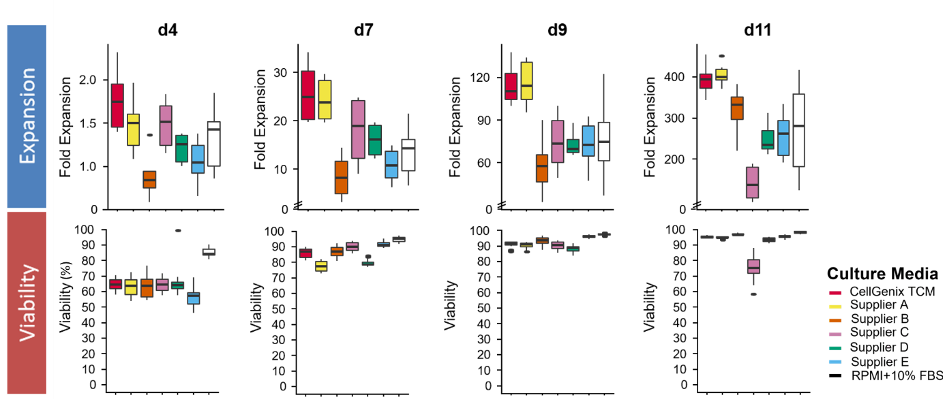
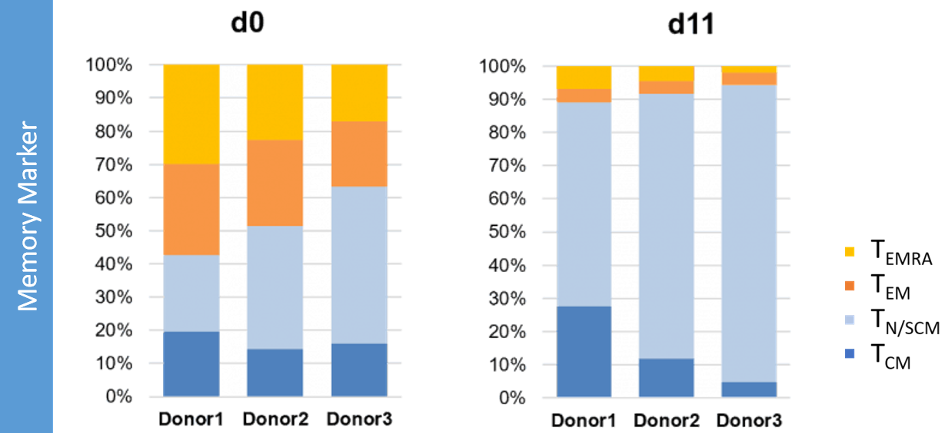
Promotion of a central memory and early differentiated memory T-cell phenotype
During T-cell culture in CellGenix TCM a ‘young’ phenotype of naïve T-cells/memory stem T-cells (TN/SCM cells) or central memory T-cells (TCM cells) is acquired. These phenotypes represent the most undifferentiated memory T-cell phenotypes, meaning that they have the highest proliferation potential, self-renewal properties and longer survival rates.
It has been shown that the engraftment and persistence of T-cell products in patients is associated with a TCM phenotype, rather than more differentiated memory phenotypes [1]. TSCM are considered as even less differentiated cells with potentially even better persistence. It has been reported that for CD19 CAR-T cells the expansion of patient-infused cells is correlated with the frequency of CD8+ TSCM-like cells [2]. It is therefore beneficial for your T-cell therapy product to obtain a central memory and early differentiated memory T-cell phenotype.
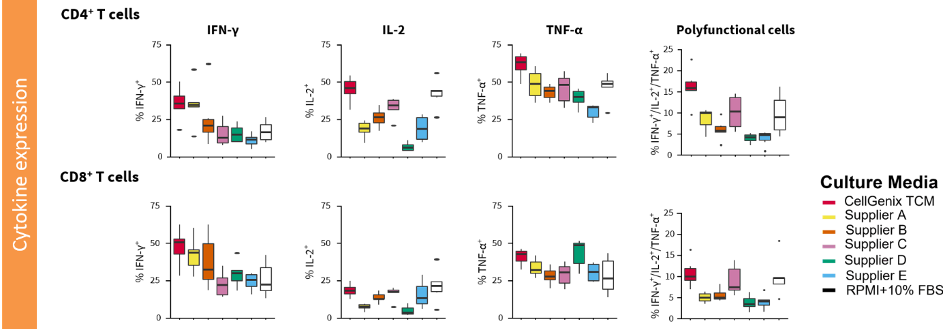
High proportion of cytokine producing cells including polyfunctional cells
An important aspect of T-cell functionality is the secretion of cytokines such as interleukin-2 (IL-2), interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α). After cultivation in CellGenix TCM a high proportion of cytokine producing cells is obtained including polyfunctional cells (expressing more than one cytokine).
A recent report suggests that the combination of cell frequency and cytokine production levels of polyfunctional CD19 CAR-T cells is correlated with the clinical outcome in patients with non-Hodgkin lymphoma [3]. Obtaining cytokine secreting T-cells, in particular polyfunctional cells, could therefore have a positive effect on the therapeutic response of your T-cell therapy product.
Interested to learn more about CellGenix TCM GMP-Prototype?
Read on: cellgenix.com/tcm
Go to our Spotlight homepage to see other amazing content, updated weekly!
References
- Schmueck-Henneresse M, Omer B, Shum T et al. Comprehensive
approach for identifying the T cell subset origin of CD3 and CD28
antibody—activated chimeric antigen receptor—modified T cells. J Immunol. 199(1), 348—362 (2017). - Xu Y, Zhang M, Ramos C et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are pre- served by IL-7 and IL-15. Blood. 123(24), 3750—3759 (2014).
- Rossi J, Paczkowski P, Shen Y et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 132(8), 804—814 (2018).
Find out more in these top picks from the Editor:
- How to choose the best medium for CAR-T manufacturing
- CAR-T mesh shrinks ovarian tumors in mice
- Preparing your raw materials for commercial manufacture: an interview with Bernd Leistler