An eye on organoids: organoids and their use in retinal degeneration
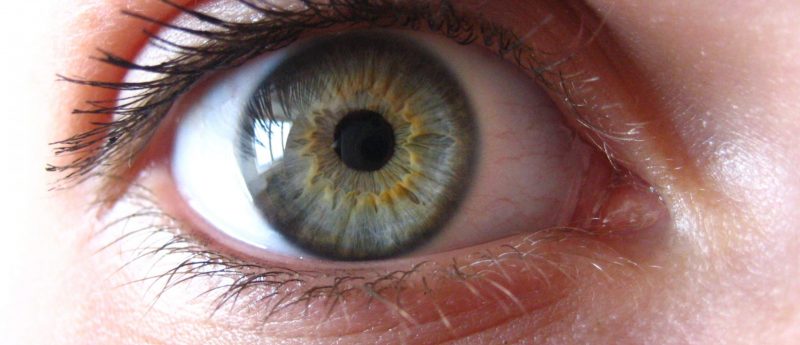
Tahani Baakdhah provides an overview of organoids and gives us an insight into her own work on bioreactors for retinal organoids. A new era of personalized medicine is on the horizon thanks to modern technology, which allows scientists to use patients’ stem cells to make different three-dimensional (3D) mini organs called ‘organoids’. Inside these organoids, cells assume organization similar to that found in the original organ they represent. This self-organization is the result of a multitude of instructive cues originating from the extracellular matrix and the surrounding culture media, as well as signals from other cell types within the organoid...