Cryoport

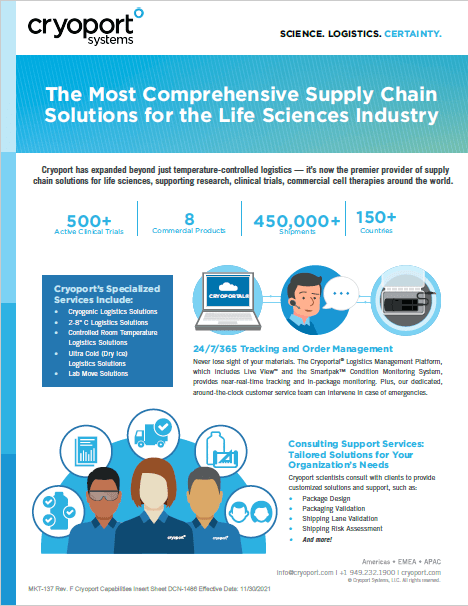
Cryoport Systems is the industry’s most trusted provider of temperature-controlled logistics and supply chain solutions for life sciences industry, serving biopharmaceutical, reproductive medicine and animal health organizations around the world. Cryoport Systems’ proprietary Cryoport Express® dry vapor shippers, innovative C3™ Refrigerated & CRT solutions, Cryoportal® logistics management platform, leading-edge Smartpak II® condition monitoring system and geo-sensing technology, paired with unparalleled cold chain expertise and 24/7/365 customer support, make Cryoport Systems the end-to-end logistics and supply chain partner that you can trust. Cryoport Systems is proudly supporting 654 active clinical trials and 10 commercial therapies in regenerative medicine.
|
|
Cryoport, Inc. announces the opening of their new 21,000 square feet facility expanding their presence in Houston, Texas. The Global Supply Chain Center will enable Cryoport and CRYOGENE to service the life sciences research sector in the surrounding metropolitan area. |
 |
In early 2022, Cryoport successfully acquired French biological and chemical sample management service company, Cell&Co BioServices. The acquisition intends to branch into the growing cell and gene therapy industry in Europe and ultimately enrich their Global Supply Chain Network. Read more about the acquisition in this article. |
 |
In this interview, Cryoport Systems’ CEO, Dr. Mark Sawicki, highlights the importance of standardization in the cell therapy development process for facilitating future growth in the industry. He delves into how standardizing collection, processing, storage and distribution of therapies are necessary for this expected growth. |
 |
Cyroport Systems expand into BioServices with the opening of their Morris Pains facility (NJ, USA). Strategically positioned in New Jersey, the state has the nation’s highest concentration of scientists and engineers per square mile. Read more about the new location and the supply chain management services on offer in this article. |
 |
Cryoport, Inc. announces a new strategic partnership with Syneos Health. The new partnership will utilize the full suite of clinical development services offered by Syneos Health with IntegriCell™, Cryoport’s platform providing standardized apheresis collection, cryopreservation, storage and distribution. |
 |
The Future of Life Sciences Supply Chain Industry: 4G/5G Network Technology AdoptionAs the United States pioneers the transition from 2G/3G networks to their 4G/5G successors, carrier services are set to encounter capacity and connectivity challenges. But how will this transition impact active shipment monitoring in the life sciences supply chain industry and could this update ultimately resolve current supply chain issues? Discover how industry experts Cryoport Systems are using this technology to stay ahead of the curve in this article with COO, Phil Wilson. |
 |
Mergermarket: Cryoport Continues Opportunistic Acquisition Drive to Build Upon Current MarketsCryoport continues to look out for opportunistic acquisitions as the foundation of their strategic business model. Their current interests lie in diversifying their portfolio among packaging, bio-storage, specialist software platforms or specialist logistic companies. Find out more about Cryoport’s recent history of opportunistic acquisitions in this article. |
 |
Reshaping The Pharmaceutical Supply Chain – Contract Pharma Q&AWith the global rollout of COVID-19 vaccines well on the way, the need for a temperature–controlled logistics provider has only been exaggerated. In this article, Mark Sawicki, CEO of Cryoport Systems, discusses how the pandemic has disrupted the pharmaceutical supply chain industry and looks forward to approvals for new cell and gene therapies. |
 |
ISO 21973: Standardizing Best Practices for Transporting Cell and Gene TherapiesAfter 3 years in the making, ISO 21973 is here to supply a set of robust best practices for the transportation of cell and gene therapies. Discover more on the new guidance from Mark Sawacki, CEO of Cryoport Systems, and Rob Jones, VP of BioServices, in this article for Pharma’s Almanac. |
 |
Cryoport and Cell Matters Collaboration Aids in the Standardization of Cell Therapy Supply Chain ProcessesEstablishing a reliable, low risk transportation method for leukopaks is essential for continuing advancements in autologous cell therapies. Cryoport’s recent partnership with Cell Matters has exploited the benefits of cryogenic refrigeration in maintaining viability and quality of cells. Discover more on how their collaboration has driven standardization in the supply chain with this article by Pharmaceutical Commerce. |
 |
|
 |
|
|
|
|
 |
|
 |
|
 |
|
 |
|
 |
|
|
|
|
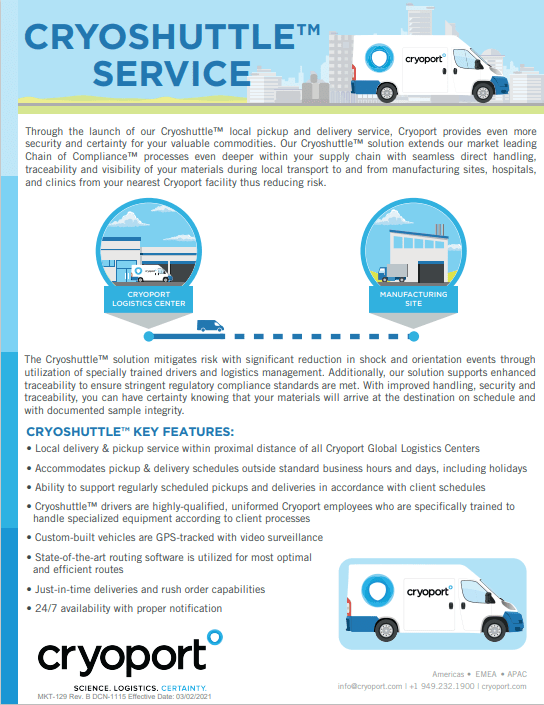
 |
|
 |
|
 |
|
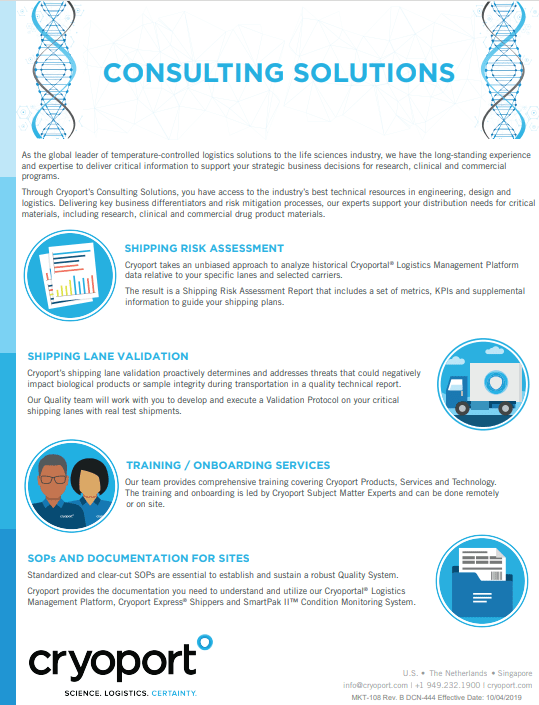
 |
|
 |
|
 |
Are you ready for the upcoming data network transition? Here’s everything you need to know>>> |
 |
2022 updates to the Harmonized System (HS) and Harmonized Tariff Schedule (HTS) |
 |
Cryoport Systems & CRYOPDP Synergies provide integrated and flexible temperature-controlled supply chain solutions for the life sciences |
 |
Introducing Cryoport BioServices in Morris Plains, NJ ─ A strategic & prime location for supporting the life sciences |
 |
Courier Agnostic – Cryoport Systems’ great advantage |
 |
What the new ISO 21973 guidance means for cell and gene therapy developers |
 |
|
Upcoming data management transactions and their impact on advanced therapy supply chains
|
A Leap Forward in Standardizing the Regenerative Medicine Supply Chain |
The Regenerative Medicine Supply Chain: Anticipate New Compliance Standards Now or Risk Falling Behind |
Biostorage |