Creating 3D ‘asteroids’: neural stem cell organoids
In this article, Geoffrey Potjewyd (University of Manchester, UK) reviews a recent report from Stem Cell Reports.
Geoffrey Potjewyd1
1Regenerative Medicine & Neuroscience PhD student, University of Manchester (UK)
Organoids are 3D spheres of aggregated cells which can be grown to replicate 3D tissue architecture. This offers advantages to 2D culture systems where cells are often grown on stiff plastic, limiting cells to planar growth and not replicating the 3D microenvironment of living tissue.
The development of organoids has been utilized to tissue engineer 3D ‘mini-brains’ for research purposes, which can develop into different layers of brain tissue. A limiting factor to the majority of these mini-brain cultures is that they only consist of one cell type — with astrocytes only forming after a long period of time — which doesn’t replicate the milieu of neurons, glial cells (astrocytes, microglia, oligodendrocytes) and vasculature present in the brain.
A multi-institute research group has developed a neural organoid model which comprises neurons and astrocytes, which they have called ‘asteroids’. This asteroid tissue organoid allows for the investigation of the interaction between neurons and astrocytes within a 3D microenvironment that replicates the in vivo situation better than 2D cultures; with cells allowed to interact with their surrounding environment in a 360° manner producing a more accurate tissue for experimentation. This will allow for researchers to investigate the cell-cell interactions between neurons and astrocytes for a range of different applications, between discovering how the interaction between the cells affects Alzheimer’s disease onset and progression, to how astrocytes contribute to local and long range neural processing.
Astrocytes are important cells within the brain, providing biochemical cues and important physical interactions that benefit the other cells in the brain. It is well known that the physical and biochemical interactions of astrocytes have a significant role in maintaining the vasculature of the brain (blood-brain barrier), regulation of neuronal synapses, regulation of oligodendrocyte growth and subsequent myelination of axons, as well as regulating inflammatory responses from microglia. These interactions make studying astrocytes in a physiologically relevant 3D environment like the asteroid culture important to ascertain what mechanisms contribute to disease, potentially uncovering key cellular and molecular processes as drug targets or biomarkers for diagnosis.
The group grew the neurons and astrocytes separately to allow for the exact ratio of neural cell types present in the asteroid culture to be controlled; thereby increasing the repeatability in development of the asteroids and ability to systematically upscale the asteroid growth.
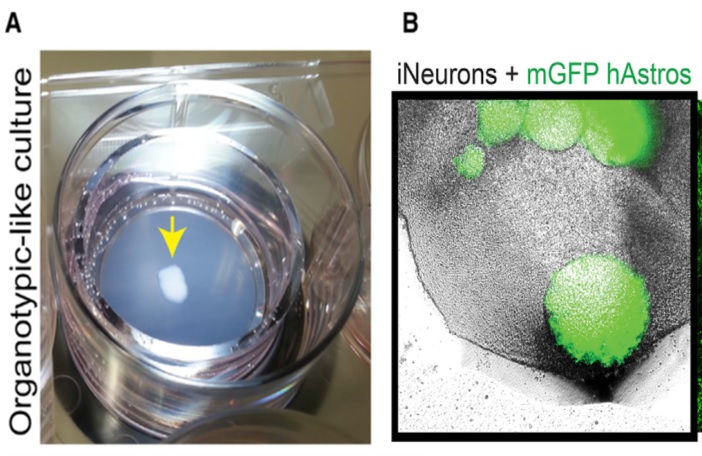
The group utilized human cells to develop the asteroid cultures, by using pluripotent stem cells to differentiate cells into the neuronal and astrocyte lineages. To differentiate the stem cells to neurons they tried out two separate tactics; one was from using a differentiation protocol with a range of biochemical triggers, whilst the other used a clonal pluripotent stem cell which had a gene inserted to increase production of a protein (Neurogenin 2) and quickly generate a homogenous population of cortical neurons. The first technique attempted had the disadvantages of creating a mixture of undifferentiated neural cells, which generate immature synapse-based astrocytes and due to the mixed nature of the growth are not able to be systematically replicated due to the inconsistent growth rates of the non-neuronal mixture.
The use of human stem cells allows for the development of clinically relevant tissues which can provide much more translatable information in relation to the human condition. This could be used to develop human models of the brain made from patients, enabling the dissection of exact disease mechanisms for specific patients or even the implementation of a stratified medicine approach to drug discovery, with patient specific tissues being tested for a response from a panel of drugs.
The authors of the paper suggested that future advancements to this work could be to include other cell types of the brain to the asteroid culture, to further probe precise cell-cell interactions within the brain. For example, through introduction of brain microvascular endothelial cells to the asteroid, it is possible to study the interaction between neurons, astrocytes and the blood-brain barrier, a critical interface in the neurovascular unit of the brain and implicated in a range of dementias and other neurodegenerative conditions. Other alternatives may be to introduce oligodendrocytes to investigate the effects of cell-cell interaction to the demyelination of neurons in multiple sclerosis.
Overall this research presents a key advance in neural organoids, with added control over the neuron and astrocyte cell populations being enabled in the asteroid model unlike other neural organoids where cell populations are variable. This allows for isolation and controlled analysis of the interactions between neurons and astrocytes. The authors pose that the asteroid culture could be utilized in determining the precise roles of astrocytes in short and long range neural circuitry, to further elucidate how astrocyte cells facilitate this key brain function. While neural circuits — or any other disease mechanism — are not analyzed in this study, the advantages of this asteroid co-culture model (lab-based, physiologically relevant 3D-microenvironment) allows for the study of this in future asteroid based models.
This also frames the importance of the interdisciplinary fields of tissue engineering and cellular biology; where the development of physiologically relevant, multicellular-human tissue can probe the underlying mechanisms of healthy and diseased tissue.
Krencik R, Seo K, van Asperen JV et al. Systematic Three-Dimensional Coculture Rapidly Recapitulates Interactions between Human Neurons and Astrocytes. Stem Cell Reports.