Mass-producing iPSC-derived cell therapies while maintaining cell quality: an interview with Kevin Alessandri, TreeFrog Therapeutics
In this interview, Kevin Alessandri (TreeFrog Therapeutics, Bordeaux, France) discusses why the mass production of induced pluripotent stem cell (iPSC)-derived therapies remains a challenge, including how TreeFrog Therapeutic’s C-Stem™ encapsulation technology can help to address some of these obstacles facing the field.
Please can you introduce yourself and your organization?
I’m Kevin Alessandri, I am a physicist specialized in cell encapsulation microfluidics. I am the co-founder of TreeFrog Therapeutics with Maxime Feyeux, an expert in pluripotent stem cell (PSC) biology. We met in Geneva (Switzerland) in 2014 and invented the C-Stem™ technology the following years. We launched TreeFrog Therapeutics in late 2018 in Bordeaux (France), with the aim of providing access to safe and affordable cell therapies to millions of patients.
The iPSC technology was invented in 2006. Thus, the field of iPS-derived cell therapies is relatively young. What has changed in the industry over the last few years?
The current state of the industry has nothing to do with what it was 5 years ago. Over 50 cell therapy programs based on PSCs have reached the clinic, and the global pipeline is now populated with preclinical programs led by very well-funded biotech companies, mainly based in the USA, such as Sana Biotechnology (WA, USA), Semma-Vertex (MA, USA), BlueRock Therapeutics (NY, USA) and Century Therapeutics (PA, USA). In 2020, according to the Alliance for Regenerative Medicine (DC, USA), the cell therapy industry attracted a record USD$11 billion in financing – a +240% increase compared to 2019. So obviously, the pipeline is going to keep growing. Big pharma companies are also investing massively in the field. Novo Nordisk (Bagsværd, Denmark) invested USD$1 billion to secure a stem cell manufacturing facility in California (USA) for instance, and Japanese pharma companies such as Takeda, Sumitomo Dainippon, Astellas and Fujifilm (all Tokyo, Japan), are also building production capacities. Now, I guess it’s in everybody’s mind that the next challenge is the commercial phase: how to scale-up manufacturing, drive costs down and ensure flawless cell quality.
Why does the mass-production of iPS-derived cell therapies remain a challenge?
Well, it’s clearly the cell culture technologies that are limiting here. Up to now, the vast majority of clinical trials have been targeting indications requiring a low number of cells, such as age-related macular degeneration or Parkinson’s disease. 2D cell culture platforms enabled production of the first clinical batches, however, going commercial with this technology is challenging. In 2D, yields are impaired by high cell death rates at every passage. The system “inevitably causes genetic alterations,” as recently noted by Nobel Prize winner, Shinya Yamanaka. Overall, 2D platforms are not scalable, therefore limiting batch reproducibility and cost containment.
To prepare the commercial phase and address indications requiring a large number of cells, the industry is now moving to bioreactor-based technologies. But here again, the system poses challenges in terms of cell viability and cell quality: with stirred-tank bioreactors, impeller-induced shear stress increases as you move to larger volumes, thereby damaging the cells. To make it short, the industry is developing iPS-derived cell therapies with mass-market potential but is missing a scalable manufacturing technology to address the commercial phase.
Could you tell us more about your manufacturing technology (C-Stem) and how it helps to tackle these challenges?
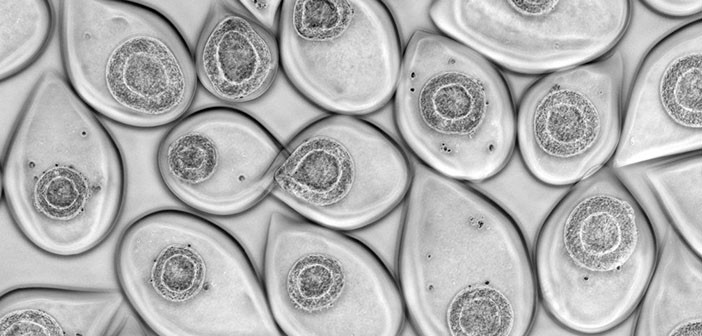
Figure 1. Representation of human iPSCs self-organized in 3D within an alginate capsule produced with C-StemTM.
C-Stem is an integrated manufacturing process to grow and differentiate stem cells in bioreactors, within a biomimetic and protective microenvironment: the capsule. The C-Stem process starts with the encapsulation of stem cells at high-throughput – 1000 capsules per second – using proprietary microfluidics. Once seeded in alginate shells, PSCs self-organize in a biomimetic 3D conformation, which ensures fast growth, with less than 2% cell death, maintenance of homogeneous pluripotency (>95%) and preservation of genomic integrity (>99%). But more importantly, capsules efficiently protect cellular content against external constraints – such as impeller-induced shear stress. As a result, encapsulated PSCs can be amplified and differentiated in large-scale bioreactors without any negative impact on cell quality. The entire process is closed, automated and delivers ready-to-graft 3D microtissues, which can be decapsulated in 60 seconds at any time by dissolving the alginate capsule. We strongly believe that this manufacturing technology is meant to become a dominant design in the industry because it is compatible with existing differentiation protocols, because it leverages bioreactors – the gold standard technology for bioproduction and because it reunites scalability with cell quality.
If your technology creates opportunities in indications requiring a large number of cells, why does your main cell therapy program target Parkinson’s disease?
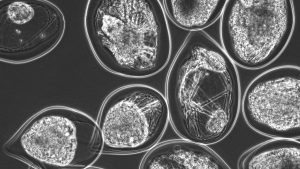
Figure 2. Microtissues of mature dopaminergic neurons differentiated from human iPSCs within an alginate capsule.
We are actually advancing cell therapies in indications requiring a lot of cells, such as heart failure, through co-development partnerships. But you’re right, it may be counter-intuitive to push a lead program in Parkinson’s disease. In fact, we decided to address this indication because the 3D graft format provides clear-cut therapeutic benefits.
All current initiatives that aim to replace dopaminergic neurons are in fact based on the transplantation of dopaminergic progenitors, and not mature neurons. It means that progenitors finish their differentiation in the patient brain, after transplantation. Results are encouraging, but there is obviously little control over the therapeutic product. The question is: why don’t we directly implant mature dopaminergic neurons instead if progenitors? Well, just because detaching mature neurons from a cell culture plate is impossible.
Overcoming this issue, C-Stem allows us to differentiate stem cells into fully mature neuronal microtissues in the capsules, within large-scale bioreactors. These mini grafts of mature dopaminergic neurons are then easily decapsulated for implantation and demonstrated better integration in the brain than progenitors administered in suspension. Our preclinical results show full motor function recovery in 8 weeks, versus 16 weeks with progenitor grafts. We also observed six-times more dopaminergic neurons with our 3D graft. Overall, our strategy ensures a faster time to effect, improves product safety and reduces production costs.
Looking to the future, what impact might this encapsulation technology have on the cell therapy industry and for patients?
Today, within the industry, our co-development partners are mainly interested in the C-Stem technology because of its benefits in terms of scalability and cell quality. Solving manufacturing and safety issues allows us to move cell therapy products along preclinical and clinical phases faster, with higher chances of reaching the market.
In the long run, we are convinced the C-Stem technology will bring outstanding benefits to patients. First, executing a scalable manufacturing model means that it becomes possible to cure not just a few hundreds, but millions of patients suffering from currently incurable diseases, such as heart failure, Type 1 diabetes, or neurodegenerative disorders. Second, in addition to making cell therapies more available and affordable, we believe that C-Stem will make them safer and more efficient in a wide range of indications. In other terms, we believe C-Stem will unleash the mass-market potential of cell therapies. Our motto, “Cell therapy for all”, encapsulates this vison.
If our readers would like more information about this technology, how can they contact you?
They can visit www.treefrog.fr and send us an email at [email protected]
Disclaimer
The opinions expressed in this interview are those of the interviewee and do not necessarily reflect the views of RegMedNet or Future Science Group.