New funding to support research into safety issues linked to gene therapy for Duchenne muscular dystrophy
The Muscular Dystrophy Association has announced two research grants to address the challenges associated with adeno-associated virus-based gene therapies for Duchenne muscular dystrophy.
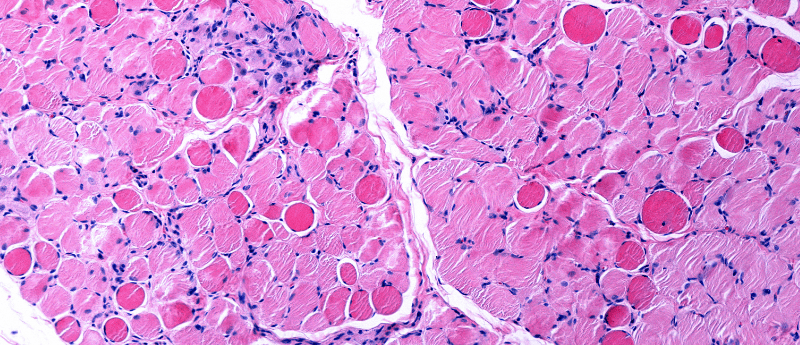
Duchenne muscular dystrophy (DMD) is a disorder characterized by progressive degeneration and weakness of cardiac and skeletal muscle. The genetic disorder is caused by mutations in the DMD gene encoding dystrophin. Adeno-associated virus (AAV)-based gene therapy has emerged as a promising therapeutic approach for DMD, with pre-clinical studies and early clinical trials yielding positive results. The approach utilizes AAV vectors to deliver exogenous micro-dystrophin, a transgene encoding a truncated but functional version of the dystrophin gene.
The decision to award two grants close to US$400,000 follows a summit hosted by the Muscular Dystrophy Association (IL, US) where concerns about the safety issues and challenges of AAV gene therapies were raised following adverse events in clinical trials.
“With these grants, we continue our longstanding collaboration with industry and academic partners to identify bottlenecks and ways to safely translate gene therapies for neuromuscular disorders,” stated Sharon Hesterlee, Chief Research Officer of the Muscular Dystrophy Association.
AAV-based gene therapy trials have recorded five serious adverse events where missing sections of the transgene has been linked to a specific patient genotype. This has resulted in the exclusion of 10-15% of patients affected by DMD from gene therapy trials.
In a bid to gain further understanding of transgene immunogenicity, US$200,000 was awarded to Jefferey Chamberlain, a professor at the Institute for Stem Cell & Regenerative Medicine at the University of Washington (WA, US). The Chamberlain lab has developed a method that identifies immunoreactive regions in the transgene, allowing the section to be redesigned before delivery. The lab will conduct a two-year study into transgene immunogenicity, identifying and redesigning the immunoreactive regions of the dystrophin transgene.
“This most recent funding will help us design the next generation of dystrophin gene replacement therapies that avoid transgene-triggered immune responses, allowing more Duchenne muscular dystrophy patients to be candidates for treatment,” said Chamberlain.
A second grant worth US$199,962 was awarded to Carrie Miceli, Professor of Microbiology, Immunology and Molecular Genetics and co-director for the Center for Duchenne Muscular Dystrophy at the University of California, Los Angeles School of Medicine (UCLA; CA, US).
Miceli commented: “Our studies promise to teach us about immune response to gene therapy, durability of micro-dystrophin expression and identify druggable pathways for improving safety and efficacy of gene therapies.“
The grant will enable the research team to use single-cell and single-nuclei sequencing methods to assess responses to gene therapies. The team will utilize muscle biopsies to analyze micro-dystrophin expression and immune response, as well as muscle health.
It is hoped that the funding will lead to the development of new dystrophin-based gene therapies that could prove to be safe, non-immunogenic and effective for the treatment of DMD.