Vertical-wheel bioreactors: a promising panacea for iPSC scale up?


Dr. Breanna Borys is a bioprocess scientist at PBS Biotech (CA, USA). Beginning her career in the Pharmaceutical Production Research Facility at the University of Calgary (Calgary, Canada), Breanna was quickly involved in several evolutions at the forefront of chemical engineering principles and stem-cell bioreactor processes. Having acquired fluency in bioreactor hydrodynamics, computational modeling, and bioprocess design specifically for pluripotent stem-cell culture, she realized that special attention was needed for the scale-up of iPSCs, leading her to work with Vertical-Wheel® bioreactors and their producers, PBS Biotech.
Here, Breanna details the vital role iPSCs will play in the future of cell therapies, the challenges associated with their scale up and how vertical-wheel bioreactors can resolve some of these challenges.
To learn more about this innovative technology, visit PBS Biotech.
What is the focus of your work?
I work in our BioLab department. This lab does a lot of internal research focused on the identification of key process input variables that impact key process outputs such as cell growth rate and yield, as well as critical quality attributes of cell products and solving bioprocess scale-up challenges. We take the knowledge we gain from our internal work to accelerate bioprocess optimization and design for our clients.
We provide contract process development services for any client limited in bioprocess or bioreactor expertise or resources, who are looking to design, test, optimize, or scale-up a stem cell bioprocess. Our teams’ extensive experience in bioprocess development and design and in-depth knowledge of various types of bioreactors allows us to systematically design experiments and analyze results to create a reproducible, robust and scalable bioprocess.
How can iPSCs help propel the allogeneic industry forward?
iPSCs hold tremendous potential in both the allogeneic and autologous regenerative medicine fields.
From an allogenic viewpoint we can screen and select superior cell lines depending on the downstream application. The field is making continuous discoveries in how different genetic and epigenetic factors can enhance proliferation, potency, and differentiation potential of iPSCs.
From an autologous viewpoint, iPSCs provide the benefits of traditional embryonic stem cells, unlimited proliferation capacity in-vitro and the ability to differentiate into all three germ layers of the human body, without the negative ethical connotations associated with embryonic stem cells and potential to avoid risk of rejection issues. This is especially important when we consider that many cell and gene therapies are often directed towards patients that are already immunocompromised and allogeneic therapies will require patients to be on immune suppressants.
So, what are the key challenges the industry is facing when it comes to clinical scale-up?
The biggest challenges I see facing the industry right now are a lack of reproducibility in bioprocess outputs, a lack of robustness in bioprocess design, and a lack of foresight into the scale-up challenges that exist when moving a small-scale process into a clinical or manufacturing scale. To expand on those topics:
- Reproducibility
You obtain a result one week and follow the same protocols, you should get the same results week after week after week after week. We see that this is very rarely the case in industry. This likely has to do with groups being unable to identify just how many bioprocess input variables exist and lacking the experience to know which ones need to be kept constant to ensure the output variables will be within range.
For example, our team has learned from experience how important certain variables in the static seed train stages are to the results we expect to achieve in the bioreactor. When the cells start to exit their exponential growth phase and enter a plateau stage in 2D, either from being over confluent or strained by another cause, they are likely to have an extended lag phase and varied growth kinetics in the following 3D stage. So, for us, we set the time frame for which the seed train harvest occurs to be very narrow to increase the reproducibility of the entire bioprocess.
- Robustness
How does the process hold up when there are changes to the process input variables. For example, if the process is carried out by a different operator, if you are using multiple cell lines, or wish to make a change to the operating agitation rate – will the protocol hold up to give a successful or comparable starting point in the results, or will any small change require an entire restructure of multiple process steps?
I think a robust process is designed when critical bioprocess variables are identified and chosen to be far away from the “edge of failure.” When testing critical input variables, large ranging studies should be carried out to determine the lower and higher ends of failure. From there, choosing values well within that window will help ensure a more robust process.
- Foresight into Scale-Up Challenges
This comes from a lack of experience and published solutions for moving small-scale stem-cell bioprocesses into large scale reactors. Our team has begun to understand at what point in the process small-scale protocols or solutions to things like feeding methods, oxygen regulation, and pH buffer capacity will no longer be feasible. It is important to consider these challenges and test what will be required protocol adjustments early in the development phases.
For example, if you are maintaining oxygen levels in a 3L bioreactor system through headspace gas addition alone, geometric differences in liquid level to headspace surface ratio in larger reactors will likely not allow for this. Alternative solutions need to be considered and tested. For example, can oxygen be sparged into the system? Can a shear protectant or antifoaming agent be added to the culture without impacting the cells?
How do fluid dynamics affect cell culture and ultimately regenerative medicine?
Fluid dynamics within a bioreactor system will have a significant impact on process outputs and cell quality attributes. From a bioprocess viewpoint an optimal bioreactor hydrodynamic environment for shear sensitive stem cells should encompass effective mixing, low power input, and scalability in design. Being able to achieve uniform mixing throughout the entire liquid volume effectively with a low power input to the impeller should result in a large range of agitation rates for which stem-cell culture is successful – giving us a better chance to operate away from the edge of failure. Having a geometry that allows hydrodynamic patterns to scale from one size reactor to the next allows operators to perform parallel testing at an economical small scale and easily scale-up optimized protocols to larger reactors without jeopardizing key process outputs and critical quality attributes of the cell products.
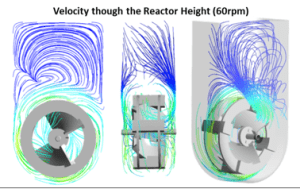
Vertical-Wheel enables optimal mixing and hydrodynamic environment
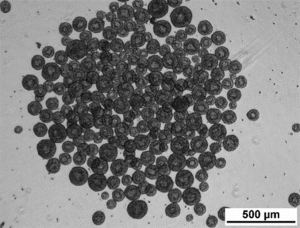
Aggregate size control and homogeneity are improved when iPSCs are cultured in Vertical-Wheel Bioreactors
From a cell quality viewpoint, it is well accepted that the hydrodynamic properties in a bioreactor can impact process outputs like cell growth rates along with quality attributes like cell viabilities, cell phenotypes, and cell potencies. For PSC cultures grown as aggregates, the hydrodynamic environment will directly impact aggregate morphology, aggregate size, aggregate size distributions, and aggregate density. These aggregate outputs impact down-steam differentiation efficiencies and lineage biases. This makes hydrodynamic characterization and control essential to regenerative medicine bioprocess designs.
What types of materials and methods are being utilized to study bioreactor hydrodynamics?
In house we utilize both experimental and computational simulation methods to characterize local and global bioreactor hydrodynamics. Computational fluid dynamic modeling allows us to study details that are impossible to obtain from experimental methodologies. For example, we can evaluate particle streamlines and assess the fluid flow patterns within the bioreactor volume overtime. We can assess the degree of radial and axial mixing within a bioreactor and pinpoint if there are any high-shear areas that could cause cell damage or any low-velocity areas that larger cell aggregates or microcarriers could settle at. We have used computational modeling to characterize our family of vertical-wheel bioreactors, to aid in product development, and to generate scale-up correlation equations based on volume-average hydrodynamic variables. These scale-up correlations allow operators to optimize many bioprocess variables at a small-scale and predict agitation rates throughout scale-up that maintain the desired hydrodynamic environment.
We have also developed, tested, and published experimental methods to obtain important empirical data on hydrodynamic variables such as power input. These experimental results are used to validate the computational models and can be used by other groups to compare different bioreactor fluid dynamic properties when computational modeling software and experts are unavailable.
What hydrodynamic properties does the vertical-wheel bioreactor have that make it better for stem cell bioprocessing?
From our computational studies and experimental cell cultures, I believe there are two key factors that make the vertical-wheel geometry so successful.
The first is fluid flow that mixes in an axial and radial direction. This takes the particles throughout the entire bioreactor volume ensuring the cells are not trapped in a high shear stress or low shear stress environment. In traditional stirred-tank bioreactors, the fluid moves in a predominately axial direction. This means that cells are not continuously flowing through the height of the bioreactor. Some aggregates may get stuck circling around the high shear impeller for an extended period of time which results in high shear and small aggregates. Other aggregates may get stuck circling around the low shear zones at the top and bottom of the liquid height, which results in low shear and large aggregates. This ultimately results in a heterogenous cell population.
The second fluid dynamic characteristic that makes the vertical-wheel geometry ideal for stem cell culture, is the narrow distribution of hydrodynamic forces throughout the bioreactor volume. When characterizing and understanding bioreactor hydrodynamics it is important to consider not just average variables such as velocity, shear stress, and energy dissipation rates, but also the distribution of these variables within the volume. Simply put, if you have an average of 10, did this occur from having a bunch of 9s and 8s (a narrow distribution), or did this occur from having a bunch of 15s and 5s (a wide distribution). Even if average hydrodynamic variables are similar between bioreactor types, differences in the distribution of these forces will relate to differences in process outputs and product quality.
What considerations should be taken when managing manufacturing costs?
Regardless of cost, cell quality and function will always remain the top priority in regenerative medicine. If cell quality is maintained, bioprocess objectives are simple: develop a process that enables the production of a clinically relevant number of cells, in a robust, efficient, and scalable manner. Resources including labor, materials, equipment, and the facility must be evaluated in the process costs. Media costs, for example, are one of the largest drivers of the overall manufacturing costs. Not only does media type need to be carefully considered, batch, fed-batch or perfusion feeding strategies should be evaluated as bioprocess engineers we look to systematically identify, test, and optimize process input variables and characterize critical variables to develop the most efficient and economical process.
When considering overall costs, a lack of reproducibility and inconsistency in critical process outputs and quality attributes are one of the leading reasons for run failure and FDA clinical denial. Taking the time to have bioprocess engineers design a bioprocess that is robust, efficient, and scalable will be the biggest cost saving in the end.
Where do you see this field in five years’ time?
In five years’ time, I hope we see some of the first pluripotent stem cell and gene therapies move through stage 3 clinical trials with approval into the health care system and an exponential increase in companies integrating engineering principles into their bioprocess designs, leading to successful manufacturing runs on target to pass through final stages of regulatory approval. Choosing the most reliable and efficient bioreactor platform and having the right mindset and skillsets in process development should help to realize this.
To learn more about this innovative technology, visit PBS Biotech.
Disclaimer
The opinions expressed in this interview are those of the interviewee and do not necessarily reflect the views of RegMedNet or Future Science Group.
This article is part of the RegMedNet Spotlight on scaling up for cell and gene therapies. Click here to view the full feature and discover expert insights on this >>>
In association with