Synopsys receives FDA 510(k) clearance for Simpleware ScanIP Medical
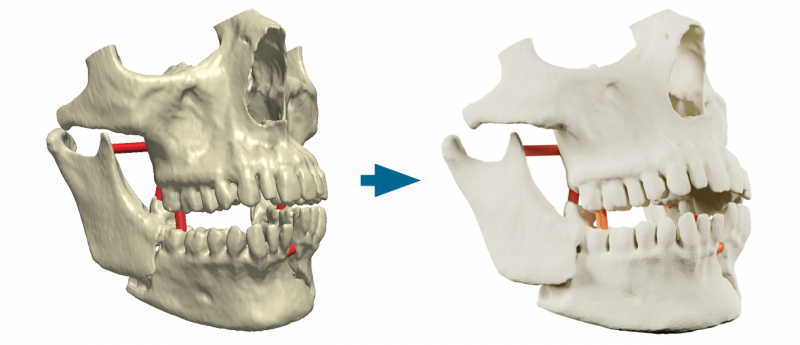
3D image processing solution Simpleware ScanIP Medical, developed by design automation company Synopsys (CA, USA), has received a 510(k) clearance from the US FDA (MD, USA). The clearance will allow the Simpleware software to be utilized for 3D-printing workflows and diagnostic decision-making. Solutions offered by the software include point-of-care 3D printing and end-to-end diagnostic 3D printing. Advantages of the Simpleware software include automated tasks and operations, the ability to bioprint high-quality anatomical models to plan surgical procedures and fully supported licensing. You may also be interested in: 3D LifePrints achieves ISO 13485:2016 certification 3D bioprinting at the nanoscale may increase...