Modular isolator utilizes hydrogen peroxide vapor for biodecontamination

Suitable for CAR-T, gene and cell therapy development, this technology could offer reduced risk and cost benefits.
At ISCT 2018 (2—5 May, Montreal, QC, Canada), Bioquell UK Ltd (UK) highlighted the suitability of its state-of-the-art Bioquell Qube aseptic workstation for the rapidly emerging CAR-T and gene and cell therapy sector. The isolator, which can be customized to suit the individual needs of cell therapy development companies, hospitals and service providers, is integrated with the company’s innovative hydrogen peroxide vapor technology for rapid bio-decontamination, reduced risk and major cost benefits.
Biocontamination in the cleanroom environment can affect the whole manufacturing process and put successful patient outcomes at risk, leading to significant financial costs and impact operational resources. As a result, regulators are specifying Good Manufacturing Practice (GMP) biologics facilities to be more proactive in terms of microbial contamination control. With operators sat at the Qube work station, product handling can be carried out within a guaranteed safe and productive ISO 5/EU Grade A environment, providing an added level of protection from potentially costly and hazardous bio-contamination.
The Qube provides an effective aseptic environment from research and development through to the manufacturing process. Utilizing hydrogen peroxide vapor allows the decontamination cycle to be started immediately, eliminating the need to reach temperature or humidity levels to begin the process with the hydrogen peroxide vapor providing a 6-log sporicidal kill over every exposed surface.
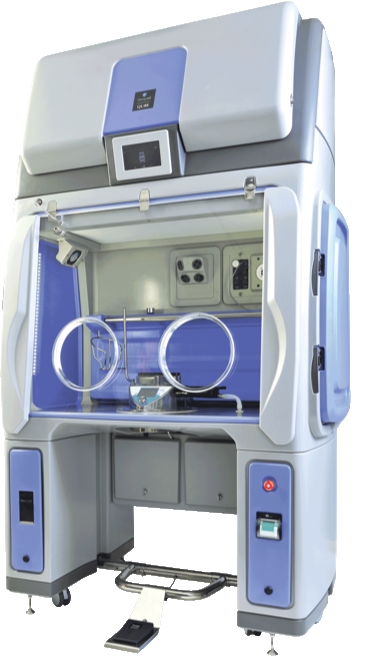
Both modular and adaptable, the Qube offers up to three chambers (two gloves in each) with optional material pass-throughs and Rapid Transfer Ports designed to meet workflow needs. It enables decontamination of materials in one chamber whilst operatives work in another, and offers aseptic-hold retention for typically seven days depending on protocols. With most organizations starting with one system, Bioquell offers the option to add chambers at a later date to suit capacity requirements.
Available with four levels of environmental monitoring for all viable and nonviable particle needs, the Qube has ability to incorporate the Merck Millipore Sigma Symbio Flex Sterility Pump and other distinctive options to ensure maximum efficiencies.
Constructed from tough and hard-wearing polypropylene, and fitting easily through standard doorways, the Qube can be installed and validated within 12 weeks with minimal disruption to operation or workflow. It offers customized validated cycles providing ISO 5/EU Grade A environment with GMP compliance and 21-CFR Part 11 software available.
Source: Bioquell press release