Serum-free media for improved clinical-scale expansion of MSCs
Mesenchymal stem cells (MSCs) are of great interest for applications in regenerative medicine. With several phase II/III clinical trials registered and more to come, reproducible culture conditions become increasingly important
Human and bovine sera are used as common supplements for the expansion of MSCs in cell culture. However, the safety profile and donor variation of serum are concerns when it comes to the translation of MSC research into pre-clinical or clinical studies. Researchers at Miltenyi Biotec have therefore developed a serum- and xeno-free MSC media formulation that is available in both research and GMP grade.
Superior expansion rates
When isolated from various tissues, MSCs are typically present in low numbers. To reach a clinically relevant scale, ex vivo expansion to 2×108 cells and more is essential. The time required for expansion largely depends on the cultivation system and requires 30 days and more when using conventional serum-containing media. Strikingly, using the novel xeno-free media formulations such as MSC-Brew GMP Medium, expansion time of clinically relevant cell numbers can be reduced by 12-13 days , thereby reducing the overall production time and costs significantly.
No attachment substrate required
A drawback of many serum-free MSC media formulations is the requirement for special cell attachment substrates. Composed of serum components, fibronectin, or proprietary attachment factors, these attachment substrates facilitate the adherence of freshly isolated MSCs to the cell culture plastic. They can only be omitted once the cultured MSCs produce sufficient amounts of extracellular matrix components endogenously. However, attachment substrates used for cell manufacturing represent additional raw/ancillary materials that require approval by the relevant regulatory bodies.The StemMACSâ„¢ MSC Expansion Media Kit XF and its corresponding MSC-Brew GMP Medium have been developed for efficient culture without the need for external substrates.
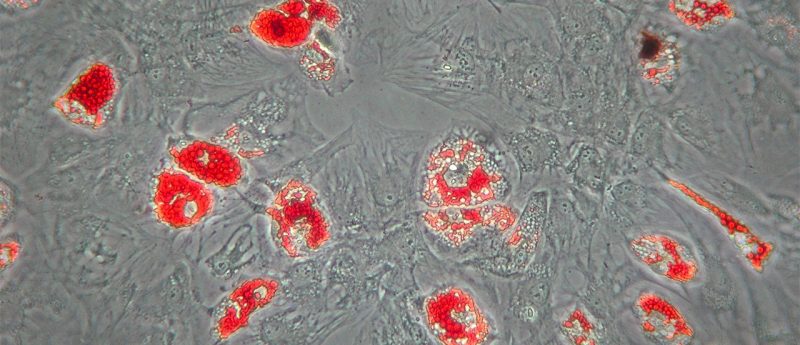
Full potential for differentiation and immunomodulation
Retaining full differentiation potential and immunomodulatory properties is key when expanding primary MSCs for clinical applications. Optimal culture conditions that support well proliferating MSCs, allow low passage numbers thereby reducing the risk for mutations or abnormalities during ex vivo expansion. MSCs that have been cultured in MSC-Brew GMP Medium, were shown to retain their potential for T cell suppression, as well as differentiation into adipocytes, osteoblasts, and chondrocytes.
Read on:
Scientific poster: Expansion of primary MSCs using MSC-Brew GMP Medium
On-demand webinar: Clinical scale cultivation of MSCs
Request a sample of MSC-Brew GMP Medium