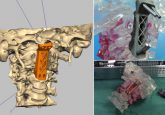
New guidance on 3D printed medical devices from FDA

Following draft guidance released in May and the review of over 100 3D printed products, the FDA has released further guidance for users. Building on earlier draft guidance on the manufacture of medical devices through 3D printing, the FDA has announced further guidelines intended to help manufacturers when submitting 3D printed devices for approval. The guidelines include advice on materials, software and device testing, particularly highlighting information the FDA will require in a submission. Figure 1: The overall 3D printing process and the related sections that are covered in this guidance Compared to the previous iteration of these guidelines from...