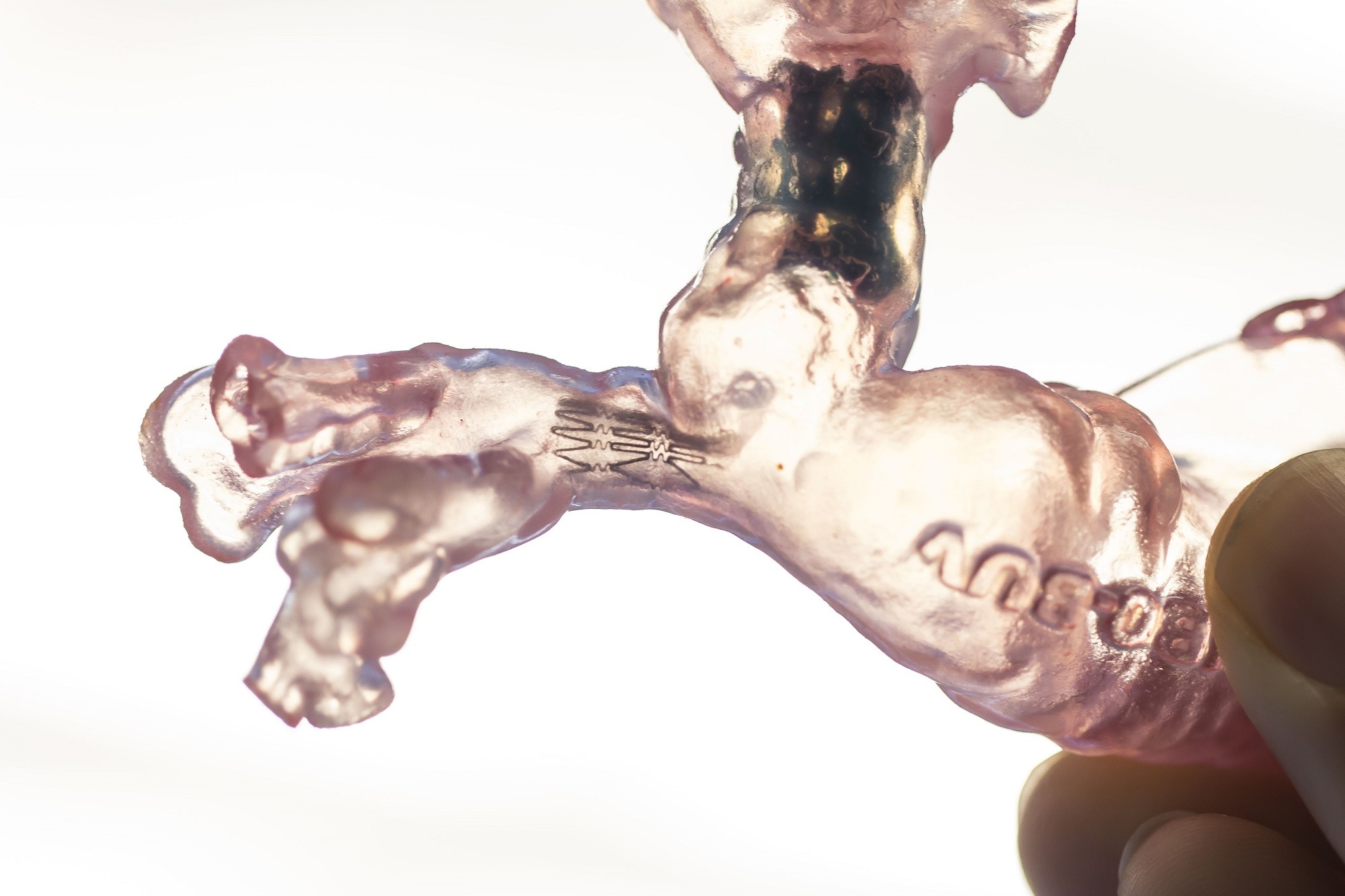
3D bioprinted human liver and kidney tissue applicable for drug toxicity testing
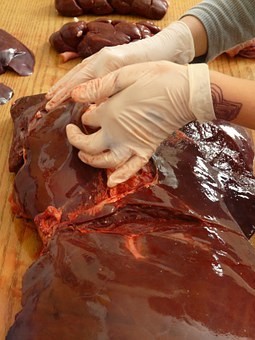
Organovo will present data demonstrating how 3D bioprinted human liver and kidney tissue can be utilized in drug testing at the 56th Annual Meeting of the Society of Toxicology and ToxExpo.
Data collected by Organovo Holdings, Inc (CA, USA) demonstrates how 3D bioprinted human liver and kidney tissue can successfully be utilized for assessing drug safety and detecting multiple clinically-relevant modes of liver injury and kidney toxicity. Organovo will present this data over eight presentations at the Society of Toxicology’s 56th Annual Meeting and ToxExpo, March 12-16, 2017 in Baltimore (MD, USA). In particular, the presentations will illustrate the wide range of applications of Organovo’s ExViveTM 3D Bioprinted Human Liver and Kidney Tissues.
“The unique ability of our human liver model to reveal mechanisms of action for drug-induced livery injury and model key aspects of chronic, progressive liver diseases such as fibrosis continues to put us at the forefront of in vitro human tissue modelling,” explained Sharon Presnell, chief scientific officer at Organovo.
“The commercial launch of our kidney proximal tubule model, in addition to our recent peer-reviewed publication highlighting its potential to become a key preclinical model for in vitro kidney toxicity testing, demonstrates our commitment to delivering novel tissue models using our platform technology,” continued Presnell.
The data presented will demonstrate that 3D bioprinted tissue can be used for many applications, including differentiating high-risk compounds from low-risk, identifying mechanisms of toxicity and assessing subsequent tissue-level recovery.
“Our powerful and versatile technology platform delivers 3D bioprinted tissues that provide an accurate, predictive and reproducible model of human liver and kidney biology for preclinical toxicity testing,” commented Paul Gallant, general manager at Organovo.
“With a growing list of applications and use cases driving market adoption including investigative toxicology, evaluation of different compound modalities and fibrosis modelling for drug discovery, demand for our drug safety testing services has been growing since its introduction, and long-term market adoption is expected to be robust given the significant gap it closes against traditional preclinical models,” concluded Gallant.
Source: