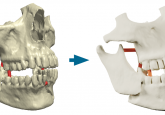
3D LifePrints achieves ISO 13485:2016 certification
Medical 3D printing and technology company, 3D LifePrints (Liverpool, UK), announced their achievement of an ISO 13485:2016 certification for its quality management system. As an internationally recognized standard of excellence, the ISO 13485:2016 keeps strict guidelines and standards for the medical device industry. Certified organizations are required to maintain quality and compliance levels specific to the design, manufacture and distribution of medical devices. 3D LifePrints’ digital platform termed Embedmed™, which includes the design and manufacture of sterilizable devices within a controlled environment, is the central design underpinning the certification. With Embedmed™ certified, timelines for the delivery of medical devices can...