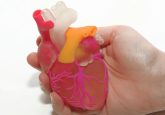
Behind the article: a 3D bioprinting exemplar of the consequences of the regulatory requirements on customized processes

In this interview, author Nick Medcalf, Professor of Regenerative Medicine Manufacture, Loughborough University (UK) discusses the advantages of 3D printing and a trend in personalized medicine.
Additive manufacturing for 3D medicinal products and devices is gathering momentum, but are existing frameworks sufficient or appropriate? In an Open Access Special Report from Regenerative Medicine, Paul Hourd et al. from Loughborough University (UK) use a 3D printed prosthetic nasal implant as a hypothetical exemplar of a 3D functional living construct to discuss the regulatory challenges posed by 3D bioprinting and its ability to create customized cell-scaffold products, which are personalized by geometry and constituent cells.
In this interview, second author Nick Medcalf, Professor of Regenerative Medicine Manufacture, Loughborough University (UK) discusses the advantages of 3D printing and a trend in personalized medicine.
What drew you to work with 3D printing?
It provides the best example of the way that healthcare product innovation is challenging the current regulations and changing the way that businesses must work with clinics to provide patient access to novel personalized treatments.
What is your greatest achievement/breakthrough so far?
Achievement rather than breakthrough: I concentrate on the operational aspects of translating these inventions to commercially-valuable manufactured goods. The interesting work in progress is all about finding new business models that keep costs down and provide early access for patients by integrating the supply with the hospital services. This is a general trend for personalized medicines.
If you weren’t working with 3D printing, what would you be doing?
This will be answered shortly! I move in September to Innovate UK to help deploy the Treasury funding for advanced therapeutics at the academia-industry interface. It’s all about getting more of the advanced therapies safely and economically to market.
Read the article now
Hourd P, Medcalf N, Segal J, Williams DJ. A 3D bioprinting exemplar of the consequences of the regulatory requirements on customized processes. Regen. Med. 10(7), 863—883 (2015).