Combining human intestinal organoids with organ-on-chip technologies for inflammatory bowel disease: an interview with Robert Barrett, PhD
In this interview, Robert Barrett (Cedars-Sinai Medical Center, LA, USA) provides us with an overview of his exciting work, which combines human intestinal organoids and organ-on-chip technologies to learn more about inflammatory bowel disease. In addition to this, he also discusses what factors require consideration when selecting a physiologically relevant extracellular matrix (ECM) for this technology, including the fantastic results he’s seen from using Cultrex™ UltiMatrix™.
Robert Barrett obtained his PhD in Neuroscience at University College Cork in Ireland and subsequently moved to Cedars-Sinai Medical Center (CA, USA) in 2007 to study the antiviral immune response in the brain. In 2009, Barrett’s research focus switched to gastroenterology when he joined the F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute (Cedars-Sinai Medical Center) and in 2011, he began to develop new models to study what role the intestinal epithelium played in inflammatory bowel disease. His research now utilizes an array of innovative technologies including induced pluripotent stem cells, human intestinal organoids and small microengineered chip technology.
What inspired you to work with organ-on-chip technology and how does it feature in your current research?
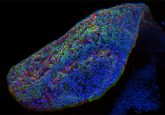
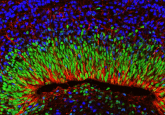
This is a great question and there are four main points for this. While intestinal organoids are really nice to look at, there are a lot of technological difficulties associated with them. First, organoids come in all different shapes and sizes, which makes it really difficult to standardize your experiments. Second, they are polarized, which means that a lot of things that we are interested in are on the interior of the organoids. For example, if you’re interested in how the microbiome interacts with the intestinal epithelium, you would have to microinject microbes into the inside of the organoids, and this is really difficult to do. Third, as they are embedded in matrix gel, it’s really difficult to do co-culture experiments. For example, if you’re interested in immune cell–intestinal epithelial cell interactions, it’s very challenging to do it in a standardized manner in the organoid system. Finally, it really allows you to carry out multicellular experiments. That is, if you want to see interactions between the microbiome, the intestinal epithelium and immune cells, the chip is a platform that allows you to carry this out in a reproducible and standardized manner.
How can your choice of reagents and technology contribute to the quality and consistency of your results (final product)?
These are very sensitive technologies and I have learnt the hard way that if you deviate at all from the protocol, or use substandard reagents or equipment, then this system will not work. You have to be certain that everything is right – that is, the quality of your cytokines and your reagents have to be to a very high standard.
What factors require consideration when selecting a physiologically relevant ECM for this technology?
There are two major factors that I can think of. The first is to ensure cell survival because most cells require a matrix to grow on. Second is to give structural support to the intestinal organoids (i.e., they must be embedded in something that allows them to maintain their 3D shape). A complex 3D structure of organoids requires a robust ECM to grow – if the organoids touch the base of the dish, they can sometimes die or will not retain their 3D shape.
What are the biggest challenges that researchers face when using various ECMs?
There are two main challenges. The first one is related to consistency – we still haven’t found a fully defined ECM that works or is cost-effective, which results in a lot of batch-to-batch variability and makes results difficult to interpret. The second relates to obtaining the ECM in large quantities at a time; sometimes it can be difficult to get those orders.
Can you tell us about your successes with Cultrex UltiMatrix and how this ECM has helped to overcome some of these challenges?
Yes, absolutely. I have used UltiMatrix for two main purposes. The first is to culture induced pluripotent stem cells and I’ve noticed that stem cells culture really well in this. The second thing I use it for is to culture iPSC-derived human intestinal organoids, and the organoids looked absolutely fantastic in it! I’ve been very pleased with the product – it’s very consistent and I’ve had no problems with it. It also appears to be readily available in large quantities, which is obviously very good for what I’m trying to achieve.
In your opinion, how translatable and scalable are these organ-on-chip platforms?
One major thing is having a personalized medicine approach. In the past, we had to use cell lines whereas now we can get patient-specific cells onto a chip and we’re almost getting away from organoids, which as I mentioned above can be technically challenging to deal with. Organ-on-chip technology can really allow us to standardize experiments and it also provides us with a platform to not only see how the tissue of interest works on its own, but also allows us to study the microbiome and immune cell interactions as well.
Lastly, in what ways do you predict organ-on-chip technology will evolve over the next 5–10 years?
There are a number of things here. First, I believe the cost for organ-on-chip technology must come down. However, I do believe that once the technology is developed, people are always up for making it cheaper and more efficient, and hopefully this develops over the next 5–10 years.
Other elements to consider include the quality of cells that go into the chip and having a more standardized approach in general. At the moment, organoid technology can be described as the ‘Wild West’ – everybody uses it in a slightly different way. They use different media, they passage at different times, etc. There’s no real way to standardize cells and that means it’s really difficult to reproduce other people’s results. Thus, I think the standardized approach to organoid generation and propagation is a big one.
Lastly, I think we will see more people putting two tissue types together. For example, I always get asked about liver–intestine interactions and I believe we will see a lot more of this, where multi-cell types from different organs are interacting at the chip system (i.e., combining a liver chip with an intestine chip).
Interested in learning more about Cultrex™ UltiMatrix™? Find out more here >>>
Disclaimer
The opinions expressed in this interview are those of the interviewee and do not necessarily reflect the views of RegMedNet or Future Science Group.
In association with:
You might also like: